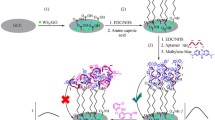
Abstract
The importance of understanding the mercury (II) ion interactions with thymine-rich DNA sequences is the reason for multiple comparative investigations carried out with the use of optical detection techniques directly in the depth of solution. However, the results of such investigations have limited applicability in the interpretation of the Hg2+ binding phenomenon by DNA sequences in thin, interfacial (electrode/solution), self-organized monolayers immobilized on polarizable surfaces, often used for sensing purposes in electrochemical biosensors. Overlooking the careful optimization of the measurement conditions is the source of discrepancies in the interpretation of the registered electrochemical signal. In this study, the chosen effects accompanying the efficiency of surface related recognition of Hg2+ by polyThymine DNA sequences labelled with methylene blue were investigated by voltammetry, QCM and spectro-electrochemical techniques. As was shown, the composition of the biosensing layer and buffers or the analytical procedures have a significant impact on the registered electrochemical readout which translates into signal stability, the biosensor’s working parameters or even the mechanism of detection. After elucidation of the above factors, the complete and ready-to-use biosensor-based analytical solution was proposed offering subpicomolar mercury ion determination with high selectivity (also in aqueous real samples), reusability, and high signal stability even after long-term storage. The developed procedures were successfully used during the miniaturization process with self-prepared (PVD) elastic transducers. The obtained sensor, together with the simplicity of its use, low manufacturing cost, and attractive analytical parameters (i.e., LOD < < Hg2+ WHO limit) can present an interesting alternative for on-site mercury ion detection in environmental samples.







Similar content being viewed by others
Data availability
Data are available on request.
References
Wu Y, Mao Y, Liu G, et al (2023) Analytical methods, occurrence, fate, and toxicity of ethylmercury in the environment: review and outlook. Rev Environ Contam Toxicol 261:. https://doi.org/10.1007/s44169-023-00037-x
World Health Organization - Mercury and health
Suvarapu LN, Seo Y-K, Baek S-O (2013) Speciation and determination of mercury by various analytical techniques. Rev Anal Chem 32:. https://doi.org/10.1515/revac-2013-0003
Zhu C, Yang G, Li H, et al (2015) Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal Chem 87:. https://doi.org/10.1021/ac5039863
Katz S (1952) The reversible reaction of sodium thymonucleate and mercuric chloride. J Am Chem Soc 74:. https://doi.org/10.1021/ja01129a023
Torigoe H, Ono A, Kozasa T (2010) Hg II Ion specifically binds with T:T mismatched base pair in duplex DNA. Chem – A Eur J 16:. https://doi.org/10.1002/chem.201001171
Miyake Y, Togashi H, Tashiro M, et al (2006) Mercury II -mediated formation of thymine−Hg II −thymine base pairs in DNA duplexes. J Am Chem Soc 128:. https://doi.org/10.1021/ja056354d
Wu J, Li L, Shen B, et al (2010) Polythymine oligonucleotide-modified gold electrode for voltammetric determination of mercury(II) in aqueous solution. Electroanalysis 22:. https://doi.org/10.1002/elan.200900441
Saidur MR, Aziz ARAA, Basirun WJ (2017) Recent advances in DNA-based electrochemical biosensors for heavy metal ion detection: A review. Biosens Bioelectron 90:125–139. https://doi.org/10.1016/j.bios.2016.11.039
Ziółkowski R, Jarczewska M, Górski Ł, Malinowska E (2013) Oligonucleotide-based electrochemical biosensor for Hg2+ using methylene blue as a redox indicator. J Electrochem Soc 160:. https://doi.org/10.1149/2.061309jes
Guerreiro G V., Zaitouna AJ, Lai RY (2014) Characterization of an electrochemical mercury sensor using alternating current, cyclic, square wave and differential pulse voltammetry. Anal Chim Acta 810:. https://doi.org/10.1016/j.aca.2013.12.005
Paleček E, Bartošík M (2012) Electrochemistry of nucleic acids. Chem Rev 112:. https://doi.org/10.1021/cr200303p
Szymczyk A, Soliwodzka K, Moskal M, et al (2022) Further insight into the possible influence of electrode blocking agents on the stem-loop based electrochemical DNA sensor parameters. Sensors Actuators B Chem 354:. https://doi.org/10.1016/j.snb.2021.131086
Zhang X, Huang C, Jiang YY, et al (2018) Structure-switching electrochemical aptasensor for single-step and specific detection of trace mercury in dairy products. J Agric Food Chem 66:. https://doi.org/10.1021/acs.jafc.8b03259
Chen DM, Gao ZF, Jia J, et al (2015) A sensitive and selective electrochemical biosensor for detection of mercury(II) ions based on nicking endonuclease-assisted signal amplification. Sensors Actuators B Chem 210:. https://doi.org/10.1016/j.snb.2014.12.114
Zhu Z, Su Y, Li J, et al (2009) Highly sensitive electrochemical sensor for mercury(II) ions by using a mercury-specific oligonucleotide probe and gold nanoparticle-based amplification. Anal Chem 81:. https://doi.org/10.1021/ac9010809
Gao C, Wang Q, Gao F, Gao F (2014) A high-performance aptasensor for mercury( <scp>ii</scp> ) based on the formation of a unique ternary structure of aptamer–Hg 2+ –neutral red. Chem Commun 50:. https://doi.org/10.1039/C4CC03275F
Ferreira CMH, Pinto ISS, Soares EV, Soares HMVM (2015) (Un)suitability of the use of pH buffers in biological, biochemical and environmental studies and their interaction with metal ions – a review. RSC Adv 5:30989–31003. https://doi.org/10.1039/C4RA15453C
Kiy MM, Zaki A, Menhaj AB, et al (2012) Dissecting the effect of anions on Hg2+ detection using a FRET based DNA probe. Analyst 137:. https://doi.org/10.1039/c2an35314h
Loaiza Ó, Campuzano S, López-Berlanga M, et al (2005) Development of a DNA Sensor Based on Alkanethiol Self- Assembled Monolayer-Modified Electrodes. Sensors 5:. https://doi.org/10.3390/s5060344
Ulianas A, Heng LY, Ahmad M, et al (2014) A regenerable screen-printed DNA biosensor based on acrylic microsphere–gold nanoparticle composite for genetically modified soybean determination. Sensors Actuators B Chem 190:. https://doi.org/10.1016/j.snb.2013.09.040
Kanchana P, Sudhan N, Anandhakumar S, et al (2015) Electrochemical detection of mercury using biosynthesized hydroxyapatite nanoparticles modified glassy carbon electrodes without preconcentration. RSC Adv 5:. https://doi.org/10.1039/C5RA11424A
Qiao W, Chiang H-C, Xie H, Levicky R (2015) Surface vs. solution hybridization: effects of salt, temperature, and probe type. Chem Commun 51:. https://doi.org/10.1039/C5CC06674C
Cooper J, Cass AEG (2004) Biosensors: a practical approach. Oxford University Press
Bard A, Faulkner L (2000) Electrochemical methods: fundamentals and applications. John Wiley and Sons Ltd
Klement WJN, Steen JD, Browne WR (2023) Selective analysis of redox processes at the electrode interface with time-resolved raman spectroscopy. Langmuir 39:. https://doi.org/10.1021/acs.langmuir.3c00633
Brown MA, Goel A, Abbas Z (2016) Effect of electrolyte concentration on the stern layer thickness at a charged interface. Angew Chemie Int Ed 55:. https://doi.org/10.1002/anie.201512025
Travers A, Muskhelishvili G (2015) <scp>DNA</scp> structure and function. FEBS J 282:. https://doi.org/10.1111/febs.13307
Porter MD, Bright TB, Allara DL, Chidsey CED (1987) Spontaneously organized molecular assemblies. 4. Structural characterization of n-alkyl thiol monolayers on gold by optical ellipsometry, infrared spectroscopy, and electrochemistry. J Am Chem Soc 109:. https://doi.org/10.1021/ja00246a011
‘diffusion layer’ in IUPAC Compendium of Chemical Terminology, 3rd ed. International Union of Pure and Applied Chemistry; 2006. Online version 3.0.1, 2019
Xiao C-Q, Huang Q, Zhang Y, et al (2020) Binding thermodynamics of divalent metal ions to several biological buffers. Thermochim Acta 691:. https://doi.org/10.1016/j.tca.2020.178721
Stellwagen NC, Bossi A, Gelfi C, Righetti PG (2000) DNA and buffers: are there any noninteracting, neutral pH buffers? Anal Biochem 287:167–175. https://doi.org/10.1006/abio.2000.4848
Delnomdedieu M, Boudou A, Georgescauld D, Dufourc EJ (1992) Specific interactions of mercury chloride with membranes and other ligands as revealed by mercury-NMR. Chem Biol Interact 81:243–269. https://doi.org/10.1016/0009-2797(92)90081-U
Kiy MM, Jacobi ZE, Liu J (2012) Metal‐induced specific and nonspecific oligonucleotide folding studied by FRET and related biophysical and bioanalytical implications. Chem – A Eur J 18:. https://doi.org/10.1002/chem.201102515
Pi K, Liu J, Van Cappellen P (2020) A DNA-based biosensor for aqueous Hg(II): Performance under variable pH, temperature and competing ligand composition. J Hazard Mater 385:. https://doi.org/10.1016/j.jhazmat.2019.121572
Smith RM, Martell AE (1989) Critical stability constants. Springer, US, Boston, MA
Acknowledgements
This work was financially supported by the Warsaw University of Technology (under the NChem4 program) and The National Centre for Research and Development under the III program TECHMATSTRATEG—Strategic research and development program ‘Modern material technologies—TECHMATSTRATEG’ no. TECHMATSTRATEG-III/0042/2019-00 and acronym ASTACUS, ‘Biopolymer materials with chemically and genetically programmed heavy metals selectivity for new generation of ultra-sensitive biosensors’.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Szymczyk, A., Popiołek, M., Krzemiński, J. et al. Identification of medium- and mechanism-related pitfalls towards improved performance and applicability of electrochemical mercury(II) aptasensors. Microchim Acta 191, 189 (2024). https://doi.org/10.1007/s00604-024-06272-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06272-w