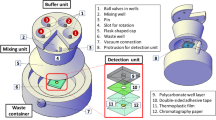
Abstract
Salmonella contamination is a major global health challenge, causing significant foodborne illness. However, current detection methods face limitations in sensitivity and time, which mostly rely on the culture-based detection techniques. Hence, there is an immediate and critical need to enhance early detection, reduce the incidence and impact of Salmonella contamination resulting in outbreaks. In this work, we demonstrate a portable non-faradaic, electrochemical sensing platform capable of detecting Salmonella in potable water with an assay turnaround time of ~ 9 min. We evaluated the effectiveness of this sensing platform by studying two sensor configurations: one utilizing pure gold (Au) and the other incorporating a semiconductor namely a zinc oxide thin film coated on the surface of the gold (Au/ZnO). The inclusion of zinc oxide was intended to enhance the sensing capabilities of the system. Through comprehensive experimentation and analysis, the LoD (limit of detection) values for the Au sensor and Au/ZnO sensor were 0.9 and 0.6 CFU/mL, respectively. In addition to sensitivity, we examined the sensing platform’s precision and reproducibility. Both the Au sensor and Au/ZnO sensor exhibited remarkable consistency, with inter-study percentage coefficient of variation (%CV) and intra-study %CV consistently below 10%. The proposed sensing platform exhibits high sensitivity in detecting low concentrations of Salmonella in potable water. Its successful development demonstrates its potential as a rapid and on-site detection tool, offering portability and ease of use. This research opens new avenues for electrochemical-based sensors in food safety and public health, mitigating Salmonella outbreaks and improving water quality monitoring.
Graphical Abstract








Similar content being viewed by others
References
Newell DG, Koopmans M, Verhoef L et al (2010) Food-borne diseases - the challenges of 20 years ago still persist while new ones continue to emerge. Int J Food Microbiol 139:S3–S15. https://doi.org/10.1016/j.ijfoodmicro.2010.01.021
Cabral JPS (2010) Water microbiology. Bacterial pathogens and water. Int J Environ Res Public Health 7:3657–3703. https://doi.org/10.3390/ijerph7103657
Craig M (2019) CDC’s antibiotic resistance threats report. Extended spectrum β-lactamase (ESBL)-producing Enterobacteriaceae. Highlights from the 2017 surveillance report. Retrieved April 29:2020
Lee KM, Runyon M, Herrman TJ et al (2015) Review of Salmonella detection and identification methods: aspects of rapid emergency response and food safety. Food Control 47:264–276. https://doi.org/10.1016/j.foodcont.2014.07.011
Pashazadeh P, Mokhtarzadeh A, Hasanzadeh M et al (2017) Nano-materials for use in sensing of Salmonella infections: recent advances. Biosens Bioelectron 87:1050–1064. https://doi.org/10.1016/j.bios.2016.08.012
Coburn B, Grassl GA, Finlay BB (2007) Salmonella, the host and disease: a brief review. Immunol Cell Biol 85:112–118. https://doi.org/10.1038/sj.icb.7100007
Onwuezobe IA, Oshun PO, Odigwe CC (2012) Antimicrobials for treating symptomatic non-typhoidal Salmonella infection. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.cd001167.pub2
Tsougeni K, Papadakis G, Gianneli M et al (2016) Plasma nanotextured polymeric lab-on-a-chip for highly efficient bacteria capture and lysis. Lab Chip 16:120–131. https://doi.org/10.1039/c5lc01217a
Mandal PK, Biswas AK, Choi K, Pal UK (2011) Methods for rapid detection of foodborne pathogens: an overview. Am J Food Technol 6:87–102
Singh G, Sithebe A, Enitan AM et al (2017) Comparison of droplet digital PCR and quantitative PCR for the detection of Salmonella and its application for river sediments. J Water Health 15:505–508. https://doi.org/10.2166/wh.2017.259
Walker DI, McQuillan J, Taiwo M et al (2017) A highly specific Escherichia coli qPCR and its comparison with existing methods for environmental waters. Water Res 126:101–110. https://doi.org/10.1016/j.watres.2017.08.032
Radhika M, Saugata M, Murali HS, Batra HV (2014) A novel multiplex PCR for the simultaneous detection of Salmonella enterica and Shigella species. Braz J Microbiol 45:667–676. https://doi.org/10.1590/S1517-83822014005000041
Wang W, Liu L, Song S et al (2015) A highly sensitive ELISA and immunochromatographic strip for the detection of Salmonella typhimurium in milk samples. Sensors (Switzerland) 15:5281–5292. https://doi.org/10.3390/s150305281
Valdivieso-Garcia A, Riche E, Abubakar O et al (2001) A double antibody sandwich enzyme-linked immunosorbent assay for the detection of Salmonella using biotinylated monoclonal antibodies. J Food Prot 64:1166–1171. https://doi.org/10.4315/0362-028X-64.8.1166
Liu J, Jasim I, Shen Z et al (2019) A microfluidic based biosensor for rapid detection of Salmonella in food products. PLoS ONE 14:1–18. https://doi.org/10.1371/journal.pone.0216873
Bhunia AK (2008) Chapter 1 - biosensors and bio‐based methods for the separation and detection of foodborne pathogens. In: Taylor SL (ed). Academic Press, pp 1–44
Wang Y, Salazar JK (2016) Culture-independent rapid detection methods for bacterial pathogens and toxins in food matrices. Compr Rev Food Sci Food Saf 15:183–205. https://doi.org/10.1111/1541-4337.12175
Silley P, Forsythe S (1996) Impedance microbiology—a rapid change for microbiologists. J Appl Bacteriol 80:233–243. https://doi.org/10.1111/j.1365-2672.1996.tb03215.x
Dhamu VN, Poudyal DC, Muthukumar S, Prasad S (2021) A highly sensitive electrochemical sensor system to detect and distinguish between glyphosate and glufosinate. J Electrochem Soc 168:057531. https://doi.org/10.1149/1945-7111/ac00f7
Cioffi A, Mancini M, Gioia V, Cinti S (2021) Office paper-based electrochemical strips for organophosphorus pesticide monitoring in agricultural soil. Environ Sci Technol 55:8859–8865. https://doi.org/10.1021/acs.est.1c01931
Li X, Gao X, Gai P et al (2020) Degradable metal-organic framework/methylene blue composites-based homogeneous electrochemical strategy for pesticide assay. Sens Actuators B Chem 323:128701. https://doi.org/10.1016/j.snb.2020.128701
Liu X, Cheng H, Zhao Y et al (2022) Portable electrochemical biosensor based on laser-induced graphene and MnO2 switch-bridged DNA signal amplification for sensitive detection of pesticide. Biosens Bioelectron 199:113906. https://doi.org/10.1016/j.bios.2021.113906
Poudyal DC, Dhamu VN, Paul A et al (2022) A novel single step method to rapidly screen for metal contaminants in beverages, a case study with aluminum. Environ Technol Innov 28:102691. https://doi.org/10.1016/j.eti.2022.102691
Poudyal DC, Dhamu VN, Samson M et al (2022) Portable pesticide electrochem-sensor: a label-free detection of glyphosate in human urine. Langmuir 38:1781–1790. https://doi.org/10.1021/acs.langmuir.1c02877
Katz E, Willner I (2003) Probing biomolecular interactions at conductive and semiconductive surfaces by impedance spectroscopy: routes to impedimetric immunosensors, DNA-sensors, and enzyme biosensors. Electroanalysis 15:913–947. https://doi.org/10.1002/elan.200390114
Davis F, Nabok AV, Higson SPJ (2005) Species differentiation by DNA-modified carbon electrodes using an ac impedimetric approach. Biosens Bioelectron 20:1531–1538. https://doi.org/10.1016/j.bios.2004.06.039
Tlili A, Abdelghani A, Ameur S, Jaffrezic-Renault N (2006) Impedance spectroscopy and affinity measurement of specific antibody-antigen interaction. Mater Sci Eng, C 26:546–550. https://doi.org/10.1016/j.msec.2005.10.007
Gai P, Pu L, Wang C, et al (2023) CeO2@NC nanozyme with robust dephosphorylation ability of phosphotriester: a simple colorimetric assay for rapid and selective detection of paraoxon. Biosens Bioelectron 220. https://doi.org/10.1016/j.bios.2022.114841
Li H, Zhao S, Wang Z, Li F (2023) Controllable preparation of 2D V2O5 peroxidase-mimetic nanozyme to develop portable paper-based analytical device for intelligent pesticide assay. Small 19:1–10. https://doi.org/10.1002/smll.202206465
Yu L, Chang J, Zhuang X et al (2022) Two-dimensional cobalt-doped Ti3C2 MXene nanozyme-mediated homogeneous electrochemical strategy for pesticides assay based on in situ generation of electroactive substances. Anal Chem 94:3669–3676. https://doi.org/10.1021/acs.analchem.1c05300
Dhamu VN, Poudyal DC, Telang CM et al (2022) Electrochemically mediated multi-modal detection strategy-driven sensor platform to detect and quantify pesticides. Electrochemical Science Advances 2:1–14. https://doi.org/10.1002/elsa.202100128
Poudyal DC, Dhamu VN, Samson M et al (2023) Pesticide analytical screening system (PASS): a novel electrochemical system for multiplex screening of glyphosate and chlorpyrifos in high-fat and low-fat food matrices. Food Chem 400:134075. https://doi.org/10.1016/j.foodchem.2022.134075
Xu B, Qi F, Zhang J et al (2016) Cobalt modified red mud catalytic ozonation for the degradation of bezafibrate in water: catalyst surface properties characterization and reaction mechanism. Chem Eng J 284:942–952. https://doi.org/10.1016/j.cej.2015.09.032
Pattnaik P (2005) Surface plasmon resonance: applications in understanding receptor-ligand interaction. Appl Biochem Biotechnol 126:79–92. https://doi.org/10.1385/abab:126:2:079
Heinrich L, Tissot N, Hartmann DJ, Cohen R (2010) Comparison of the results obtained by ELISA and surface plasmon resonance for the determination of antibody affinity. J Immunol Methods 352:13–22. https://doi.org/10.1016/j.jim.2009.10.002
Upasham S, Prasad S (2021) Tuning SLOCK toward chronic disease diagnostics and management: label-free sweat interleukin-31 detection. ACS Omega 6:20422–20432. https://doi.org/10.1021/acsomega.1c02414
Ukuku DO, Fett WF (2002) Relationship of cell surface charge and hydrophobicity to strength of attachment of bacteria to cantaloupe rind. J Food Prot 65:1093–1099. https://doi.org/10.4315/0362-028X-65.7.1093
Dickson JS, Koohmaraie M (1989) Cell surface charge characteristics and their relationship to bacterial attachment to meat surfaces. Appl Environ Microbiol 55:832–836. https://doi.org/10.1128/aem.55.4.832-836.1989
Jagannath B, Muthukumar S, Prasad S (2018) Electrical double layer modulation of hybrid room temperature ionic liquid/aqueous buffer interface for enhanced sweat based biosensing. Anal Chim Acta 1016:29–39. https://doi.org/10.1016/j.aca.2018.02.013
Munje RD, Muthukumar S, Prasad S (2017) Lancet-free and label-free diagnostics of glucose in sweat using zinc oxide based flexible bioelectronics. Sens Actuators B Chem 238:482–490. https://doi.org/10.1016/j.snb.2016.07.088
Vitarelli MJ Jr (2013) Using electrical chemical impedance spectroscopy to determine nanocapillary geometry and differential capacitance by developing a variable topology network circuit model. Rutgers The State University of New Jersey, School of Graduate Studies
Lim CY, Owens NA, Wampler RD et al (2014) Succinimidyl ester surface chemistry: implications of the competition between aminolysis and hydrolysis on covalent protein immobilization. Langmuir 30:12868–12878. https://doi.org/10.1021/la503439g
Luo X, Davis JJ (2013) Electrical biosensors and the label free detection of protein disease biomarkers. Chem Soc Rev 42:5944–5962. https://doi.org/10.1039/c3cs60077g
Chang BY, Park SM (2010) Electrochemical impedance spectroscopy. Annu Rev Anal Chem 3:207–229. https://doi.org/10.1146/annurev.anchem.012809.102211
Dhamu VN, Poudyal DC, Telang CM, et al (2022) Electrochemically mediated multi-modal detection strategy-driven sensor platform to detect and quantify pesticides. Electrochem Sci Adv 2. https://doi.org/10.1002/elsa.202100128
Krishna MS, Singh S, Batool M, et al (2022) A review on 2D-ZnO nanostructure based biosensors: from materials to devices. Mater Adv 320–354. https://doi.org/10.1039/d2ma00878e
McEnroe RJ et al (2014) CLSI. Evaluation of precision of quantitative measurement procedures; approved guideline. CLSI document EP05-A3; Clinical and Laboratory Standards Institute Wayne (PA)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Drs. Shalini Prasad and Sriram Muthukumar have a significant interest in Enlisense LLC, a company that may have a commercial interest in the results of this research and technology. The potential individual conflict of interest has been reviewed and managed by The University of Texas at Dallas and played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report, or in the decision to submit the report for publication. Portable device and technology platform is a proprietary of EnLiSense LLC.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mishra, K.K., Dhamu, V.N., Poudyal, D.C. et al. PathoSense: a rapid electroanalytical device platform for screening Salmonella in water samples. Microchim Acta 191, 146 (2024). https://doi.org/10.1007/s00604-024-06232-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06232-4