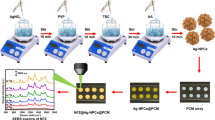
Abstract
A one-target-many-trigger signal model sensing strategy is proposed for quickly, sensitive and on-site detection of the environmental pollutant p-aminophenol (PAP) by use of a commercial personal glucose meter (PGM) for signal readout with the core–shell “loading-type” nanomaterial MSNs@MnO2 as amplifiable nanoprobes. In this design, the mesoporous silica nanoparticles (MSNs) nanocontainer with entrapped signal molecule glucose is coated with redoxable manganese dioxide (MnO2) nanosheets to form the amplifiable nanoprobes (Glu-MSNs@MnO2). When encountered with PAP, the redox reaction between the MnO2 and PAP can induce the degradation of the outer layer of MSNs@MnO2, liberating multiple copies of the loaded glucose to light up the PGM signal. Owing to the high loading capability of nanocarriers, a “one-to-many” relationship exists between the target and the signal molecule glucose, which can generate adequate signal outputs to achieve the requirement of on-site determination of environmental pollutants. Taking advantage of this amplification mode, the developed PAP assay owns a dynamic linear range of 10.0–400 μM with a detection limit of 2.78 μM and provides good practical application performance with above 96.7 ± 4.83% recovery in environmental water and soil samples. Therefore, the PGM-based amplifiable sensor for PAP proposed can accommodate these requirements of environment monitoring and has promising potential for evaluating pollutants in real environmental samples.
Graphical Abstract






Similar content being viewed by others
Data availability
The authors declare that all data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Wang CC, Wang W, Wang J, Zhang PP, Miao SD, Jin B, Li LN (2020) Effective removal of aromatic pollutants via adsorption and photocatalysis of porous organic frameworks. RSC Adv 10(53):32016–32019. https://doi.org/10.1039/d0ra05724j
Wang Y, Hu DF, Zhang ZX, Yao JM, Militky J, Wiener J, Zhu GC, Zhang GQ (2021) Fabrication of manganese oxide/PTFE hollow fiber membrane and its catalytic degradation of phenol. Materials 14(13):365. https://doi.org/10.3390/ma14133651
Benavente R, Lopez-Tejedor D, Perez-Rizquez C, Palomo JM (2018) Ultra-fast degradation of p-aminophenol by a nanostructured iron catalyst. Molecules 23(9):166. https://doi.org/10.3390/molecules23092166
Shaban SM, Moon BS, Kim DH (2021) Selective and sensitive colorimetric detection of p-aminophenol in human urine and paracetamol drugs based on seed-mediated growth of silver nanoparticles. Environ Technol Innov 22:101517. https://doi.org/10.1016/j.eti.2021.101517
Liu Y, Sheng Y, Yin YC, Ren JA, Lin XR, Zou XJ, Wang XG, Lu XG (2022) Phosphorus-doped activated coconut shell carbon-anchored highly dispersed Pt for the chemoselective hydrogenation of nitrobenzene to p-aminophenol. ACS Omega 7(13):11217–11225. https://doi.org/10.1021/acsomega.2c00093
Li M, Ding CP, Jia PD, Guo LH, Wang S, Guo ZY, Su FM, Huang YJ (2021) Semi-quantitative detection of p-aminophenol in real samples with colorfully naked-eye assay. Sens Actuator B-Chem 334:129604. https://doi.org/10.1016/j.snb.2021.129604
Santos L, Paiga P, Araujo AN, Pena A, Delerue-Matos C, Montenegro M (2013) Development of a simple analytical method for the simultaneous determination of paracetamol, paracetamol-glucuronide and p-aminophenol in river water. J Chromatogr B 930:75–81. https://doi.org/10.1016/j.jchromb.2013.04.032
Tanada N, Kageura M, Hara K, Hieda Y, Takamoto M, Kashimura S (1991) Identification of human hair stained with oxidation hair dyes by gas chromatographic-mass spectrometric analysis. Forensic Sci Int 52(1):5–11. https://doi.org/10.1016/0379-0738(91)90090-6
Feng YF, Li YG, Yu SY, Yang QR, Tong YB, Ye BC (2021) Electrochemical sensor based on N-doped carbon dots decorated with manganese oxide nanospheres for simultaneous detection of p-aminophenol and paracetamol. Analyst 146(16):5135–5142. https://doi.org/10.1039/d1an00966d
Guan HA, Zhang Y, Liu SP (2022) A novel enhanced electrochemical sensor based on the peroxidase-like activity of Fe3O4@Au/MOF for the detection of p-aminophenol. J Appl Electrochem 52(6):989–1002. https://doi.org/10.1007/s10800-022-01684-z
Narouie S, Rounaghi GH, Saravani H, Shahbakhsh M (2022) Iodine/iodide-doped polymeric nanospheres for simultaneous voltammetric detection of p-aminophenol, phenol, and; nitrophenol. Microchim Acta 189(8):267. https://doi.org/10.1007/s00604-022-05361-y
Xiao Y, Wang K, Yu X-D, Xu J-J, Chen H-Y (2007) Separation of aminophenol isomers in polyelectrolyte multilayers modified PDMS microchip. Talanta 72:1316–1321. https://doi.org/10.1016/j.talanta.2007.01.037
Zhao JJ, Liu PY, Song LJ, Zhang L, Liu ZL, Wang YQ (2021) A water stable Eu(iii)-organic framework as a recyclable multi-responsive luminescent sensor for efficient detection of p-aminophenol in simulated urine, and Mn-VII and Cr-VI anions in aqueous solutions. Dalton Trans 50(15):5236–5243. https://doi.org/10.1039/d1dt00112d
Lu XL, Wei FD, Xu GH, Wu YZ, Yang J, Hu Q (2017) Surface molecular imprinting on silica-coated CdTe quantum dots for selective and sensitive fluorescence detection of p-aminophenol in water. J Fluoresc 27(1):181–189. https://doi.org/10.1007/s10895-016-1944-7
Alatawi H, Hogan A, Albalawi I, O’Sullivan-Carroll E, Wang YN, Moore E (2022) Fast determination of paracetamol and its hydrolytic degradation product p-aminophenol by capillary and microchip electrophoresis with contactless conductivity detection. Electrophoresis 43(7–8):857–864. https://doi.org/10.1002/elps.202100347
Zhang XN, Huang XY, Wang ZL, Zhang Y, Huang XW, Li ZH, Daglia M, Xiao JB, Shi JY, Zou XB (2022) Bioinspired nanozyme enabling glucometer readout for portable monitoring of pesticide under resource-scarce environments. Chem Eng J 429:132243. https://doi.org/10.1016/j.cej.2021.132243
Zhang H, Gong ZM, Li Y, Yang FQ (2022) A simple and green method for direct determination of hydrogen peroxide and hypochlorite in household disinfectants based on personal glucose meter. Enzyme Microb Technol 155:109996. https://doi.org/10.1016/j.enzmictec.2022.109996
Li F, Li XX, Zhu NW, Li RH, Kang HB, Zhang QP (2020) An aptasensor for the detection of ampicillin in milk using a personal glucose meter. Anal Methods 12(26):3376–3381. https://doi.org/10.1039/d0ay00256a
Liu JJ, Geng ZX, Fan ZY, Liu J, Chen HD (2019) Point-of-care testing based on smartphone: the current state-of-the-art (2017–2018). Biosens Bioelectron 132:17–37. https://doi.org/10.1016/j.bios.2019.01.068
He F, Wang HJ, Du PF, Li TF, Wang WT, Tan TY, Liu YB, Ma YL, Wang YS, Abd El-Aty AM (2023) Personal glucose meters coupled with signal amplification technologies for quantitative detection of non-glucose targets: recent progress and challenges in food safety hazards analysis. J Pharm Anal 13(3):223–238. https://doi.org/10.1016/j.jpha.2023.02.005
Xiang Y, Lu Y (2013) An invasive DNA approach toward a general method for portable quantification of metal ions using a personal glucose meter. Chem Commun 49(6):585–587. https://doi.org/10.1039/c2cc37156a
Huang XT, Li JW, Lu M, Zhang WN, Xu ZM, Yu BY, Tian JW (2020) Point-of-care testing of microRNA based on personal glucose meter and dual signal amplification to evaluate drug-induced kidney injury. Anal Chim Acta 1112:72–79. https://doi.org/10.1016/j.aca.2020.03.051
Shan YK, Zhang Y, Kang WJ, Wang B, Li JH, Wu XP, Wang SY, Liu F (2019) Quantitative and selective DNA detection with portable personal glucose meter using loo;based DNA competitive hybridization strategy. Sens Actuator B-Chem 282:197–203. https://doi.org/10.1016/j.snb.2018.11.062
Ma WX, Liu MH, Xie SP, Liu B, Jiang LZ, Zhang XR, Yuan XY (2022) CRISPR/Cas12a system responsive DNA hydrogel for label-free detection of non-glucose targets with a portable personal glucose meter. Anal Chim Acta 1231:340439. https://doi.org/10.1016/j.aca.2022.340439
Cui YL, Zhao QY, Wang CQ, Jiao BN, He Y, Liu HR, Xie LYZ, Wang YW, Liu YL, Fu RJ (2023) Construction of a portable immunosensor for the sensitive detection of carbendazim in agricultural products using a personal glucose meter. Food Chem 407:135161. https://doi.org/10.1016/j.foodchem.2022.135161
Kwon D, Lee H, Yoo H, Hwang J, Lee D, Jeon S (2018) Facile method for enrofloxacin detection in milk using a personal glucose meter. Sens Actuator B-Chem 254:935–939. https://doi.org/10.1016/j.snb.2017.07.118
Cao YZ, Mo FY, Liu YH, Liu Y, Li GP, Yu WQ, Liu XQ (2022) Portable and sensitive detection of non-glucose target by enzyme-encapsulated metal-organic-framework using personal glucose meter. Biosens Bioelectron 198:113819. https://doi.org/10.1016/j.bios.2021.113819
Yang WX, Lu XH, Wang YC, Sun SJ, Liu CH, Li ZP (2015) Portable and sensitive detection of protein kinase activity by using commercial personal glucose meter. Sens Actuator B-Chem 210:508–512. https://doi.org/10.1016/j.snb.2015.01.027
Gao X, Li XY, Sun XZ, Zhang JY, Zhao YC, Liu XJ, Li F (2020) DNA tetrahedra-cross-linked hydrogel functionalized paper for onsite analysis of DNA methyltransferase activity using a personal glucose meter. Anal Chem 92(6):4592–4599. https://doi.org/10.1021/acs.analchem.0c00018
Xu CN, Lin M, Wang TY, Yao ZL, Zhang WQ, Feng XJ (2022) Colorimetric aptasensor for on-site detection of acetamiprid with hybridization chain reaction-assisted amplification and smartphone readout strategy. Food Control 137:108934. https://doi.org/10.1016/j.foodcont.2022.108934
Zhang MY, Ye J, He JS, Zhang F, Ping JF, Qian C, Wu J (2020) Visual detection for nucleic acid-based techniques as potential on-site detection methods A review. Anal Chim Acta 1099:1–15. https://doi.org/10.1016/j.aca.2019.11.056
Zhou YB, Yang S, Xiao Y, Zou Z, Qing ZH, Liu JW, Yang RH (2019) Cytoplasmic protein-powered in situ fluorescence amplification for intracellular assay of low-abundance analyte. Anal Chem 91(23):15179–15186. https://doi.org/10.1021/acs.analchem.9b03980
Wang J, Li XL, Chen HY, Xu JJ (2020) “Loading-type” plasmonic nanoparticles for detection of peroxynitrite in living cells. Anal Chem 92(23):15647–15654. https://doi.org/10.1021/acs.analchem.0c04017
Lu LL, Zhang Q, Gu Y, Li XL, Xie JJ (2022) Core-shell “loading-type” nanomaterials towards: simultaneous imaging analysis of glutathione and microRNA. Anal Chim Acta 1196:339551. https://doi.org/10.1016/j.aca.2022.339551
Yuan DS, Zhang T, Guo Q, Qiu FX, Yang DY, Ou ZP (2017) A novel hierarchical hollow SiO2@MnO2 cubes reinforced elastic polyurethane foam for the highly efficient removal of oil from water. Chem Eng J 327:539. https://doi.org/10.1016/j.cej.2017.06.144
Fu LB, Zhuang JY, Lai WQ, Que XH, Lu MH, Tang DP (2013) Portable and quantitative monitoring of heavy metal ions using DNAzyme-capped mesoporous silica nanoparticles with a glucometer readout. J Mater Chem B 1:6123. https://doi.org/10.1039/c3tb21155j
Funding
This work was supported by the National Key Research and Development Program of China (2018YFA0901300), the National Natural Science Foundation of China (Grant No. 22078149), the Natural Science Foundation of Jiangsu Province (No. BK20220002), and the Key Research and Development Program of Jiangsu Province (No. BE2023360).
the National Key Research and Development Program of China,2018YFA0901300,Jing jing Xie,National Natural Science Foundation of China,22078149,Jing jing Xie,Natural Science Foundation of Jiangsu Province,BK20220002,Jing jing Xie,Jiangsu Provincial Key Research and Development Program,BE2023360,Jing jing Xie
Author information
Authors and Affiliations
Contributions
Xiang-Ling Li: conceptualization; methodology; formal analysis; investigation; writing—original draft; visualization. Lei Zhao: investigation; writing—review and editing. Zi-Heng Wang: validation; writing—review and editing. Tian-shun Song: writing—review and editing. Ting Guo: conceptualization; writing—review and editing. Jing Jing Xie: conceptualization; project administration; funding acquisition; supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, XL., Zhao, L., Wang, ZH. et al. Core–shell “loading-type” nanomaterials enabling glucometer readout for portable and sensitive detection of p-aminophenol in real samples. Microchim Acta 191, 127 (2024). https://doi.org/10.1007/s00604-024-06204-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06204-8