Abstract
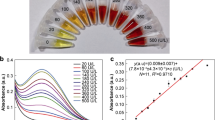
A smartphone-assisted, paper-based ratio fluorescence probe is presented for the rapid, low-cost and on-site quantification of total flavonol glycosides in Ginkgo biloba extracts (GBE). The Al3+/Eu-MOF/paper-based probe utilizes lanthanide metal-organic framework (Ln-MOF) nanoparticles immobilized on Whatman filter paper along with Al3+ for detecting flavonols, which are the hydrolyzed products of flavonol glycosides. The color change of the paper-based fluorescence image from red to orange depends on the concentration of the target analyte in the sample solution. The smartphone equipped with a red, green, blue (RGB) color detector measured the fluorescence signal intensity on the paper substrate after adding flavonol. The analytical variables affecting the performance of the probe, including the addition sequence of the aluminum nitrate solution, its concentration, that of the Ln-MOF solution, the drying time of the paper probe, the reaction time and the sensitivity parameters of the mobile phone camera (ISO), were optimized. Under optimal conditions, the Al3+/Eu-MOF/paper-based probe has good linear response in the concentration range 7 ~ 80 µg mL− 1 and a lower detection limit of 2.07 µg mL− 1. The results obtained with the paper-based ratio fluorescence probe and smartphone combination were validated by comparing them with high-performance liquid chromatography (HPLC) measurements. This study provides a potential strategy for fabricating Al3+/Eu-MOF/paper-based probe used for total flavonol glycosides determination.
Graphical abstract







Similar content being viewed by others
References
Ku YS, Ng MS, Cheng SS, Lo AW, Xiao Z, Shin TS, Chung G, Lam HM (2020) Understanding the composition, biosynthesis, Accumulation and Transport of Flavonoids in crops for the Promotion of crops as healthy sources of flavonoids for human consumption. Nutrients 12:1717. https://doi.org/10.3390/nu12061717
Gorniak I, Bartoszewski R, Kroliczewski J (2019) Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem Rev 18(1):241–272. https://doi.org/10.1007/s11101-018-9591-z
Parmenter BH, Croft KD, Hodgson JM, Dalgaard F, Bondonno CP, Lewis JR, Cassidy A, Scalbert A, Bondonno NP (2020) An overview and update on the epidemiology of flavonoid intake and Cardiovascular Disease risk. Food Funct 11(8):6777–6806. https://doi.org/10.1039/d0fo01118e
China, PCotPsRo (2020) Pharmacopoeia of the people’s Republic of China. China Medical Science and Technology Press, BeiJing, p 434
Shraim AM, Ahmed TA, Rahman MM, Hijji YM (2021) Determination of total flavonoid content by aluminum chloride assay: a critical evaluation. Lwt-Food Sci Technol 150(2):111932. https://doi.org/10.1016/j.lwt.2021.111932
Sloley BD, Tawfik SR, Scherban KA, Tam YK (2003) Quality control analyses for ginkgo extracts require analysis of intact flavonol glycosides. J Food Drug Anal 11(2):102–107. https://doi.org/10.38212/2224-6614.2718
Xu CR, Zhong WZ, Zhou Q, Zhang XC, Yang JJ, Wu YL (2017) Heterogeneity of the resistance to gefitinib treatment in a non-small cell Lung cancer patient with active epidermal growth factor receptor mutation. Thorac Cancer 8(1):51–53. https://doi.org/10.1111/1759-7714.12382
Hu J, Zhang Z (2020) Application of Electrochemical Probes based on Carbon nanomaterials for detection of flavonoids. Nanomaterials 10(10). https://doi.org/10.3390/nano10102020
Wu T, Yu C, Li R, Li J (2018) Minireview: recent advances in the determination of flavonoids by capillary electrophoresis. Instrum Sci Technol 46(4):1–23. https://doi.org/10.1080/10739149.2017.1394877
Li M, Wang D, Peng C, Wang Z (2023) Simultaneous qualitative and quantitative analysis of flavonols in Kaempferia Galangal L. and honey by machine learning-based fluorescence probe array. Sens Actuators B 378:133183. https://doi.org/10.1016/j.snb.2022.133183
Lin JH, Chen SJ, Lee JE, Chu WY, Yu CJ, Chang CC, Chen CF (2022) The detection of Mercury(II) ions using fluorescent gold nanoclusters on a portable paper-based device. Chem Eng J 430:133070. https://doi.org/10.1016/j.cej.2021.133070
Xie X, Pan M, Hong L, Liu K, Yang J, Wang S, Song Y, Wang S (2022) Carbon dots-embedded fluorescent molecularly imprinted photonic crystals hydrogel strip for accurate and selective detection of rutin in Sophora japonica products. Sens Actuators B 368:132196. https://doi.org/10.1016/j.snb.2022.132196
Bai G, Zhang T, Hou Y, Ding G, Jiang M, Luo G (2018) From quality markers to data mining and intelligence assessment: a smart quality-evaluation strategy for traditional Chinese medicine based on quality markers. Phytomedicine 44:109–116. https://doi.org/10.1016/j.phymed.2018.01.017
Peng J, Su Y, Huang FQ, Zuo Q, Yang L, Li J, Zhao L, Qi LW (2019) A simple and rapid fluorescent approach for flavonoids probe based on gold nanoclusters. J Colloid Interface Sci 539:175–183. https://doi.org/10.1016/j.jcis.2018.12.042
Xu JQ, Guo JS, Xie KF, Gao MJ, Wei R, Xin ZH, Kang YF (2022) A turn-on mitochondria-targeted fluorescent probe for detecting and imaging hydrogen peroxide in vitro and in vivo. Dyes Pigm 204:110437. https://doi.org/10.1016/j.dyepig.2022.110437
Zhang C, Yao H, Ma Q, Yu B (2021) Ultrasensitive glucose detection from tears and saliva through integrating a glucose oxidase-coupled DNAzyme and CRISPR-Cas12a. Analyst 146(21):6576–6581. https://doi.org/10.1039/d1an01385h
Dong P, Liu Y, Zhao Y, Wang W, Pan M, Liu Y, Liu X (2020) Ratiometric fluorescence sensing of copper ion and enzyme activity by nanoprobe-mediated autocatalytic reaction and catalytic cascade reaction. Sens Actuators B. https://doi.org/10.1016/j.snb.2020.127873
Zhang Z, Miao Y, Lian L, Yan G (2015) Detection of quercetin based on Al3+-amplified phosphorescence signals of manganese-doped ZnS quantum dots. Anal Biochem 489:17–24
Yan Q, Wang Y, Wang ZL, Zhang G, Shi DH, Xu HJ (2022) A novel water-soluble flavonol-based fluorescent probe for highly specific and sensitive detection of Al3+ and its application in onion and zebrafish. Spectrochim Acta Part A Mol Biomol Spectrosc 279:121384. https://doi.org/10.1016/j.saa.2022.121384
Hollman PCH, vanTrijp JMP, Buysman M (1996) Fluorescence detection of flavonols in HPLC by postcolumn chelation with aluminum. Anal Chem 68(19):3511–3515. https://doi.org/10.1021/ac960461w
Hou B, Yang S, Li B, Li G, Zheng H, Qin C, Shan G, Su Z, Wang X (2022) Construction of multi-hydroxyl/ketone lanthanide metal–organic frameworks for understanding mechanochromic luminescence and high proton conductivity. Inorg Chem Front 9(17):4376–4384. https://doi.org/10.1039/D2QI01103D
Li B, Dong JP, Zhou Z, Wang R, Wang LY, Zang SQ (2021) Robust lanthanide metal–organic frameworks with all-in-one multifunction: efficient gas adsorption and separation, tunable light emission and luminescence sensing. J Mater Chem C 9(10):3429–3439. https://doi.org/10.1039/D0TC05707J
Li PZ, Wang XJ, Zhao Y (2019) Click chemistry as a versatile reaction for construction and modification of metal-organic frameworks. Coord Chem Rev 380:484–518. https://doi.org/10.1016/j.ccr.2018.11.006
Zhang R, Zhu L, Yue B (2023) Luminescent properties and recent progress in applications of lanthanide metal-organic frameworks. Chin Chem Lett 34(2):108009. https://doi.org/10.1016/j.cclet.2022.108009
Boobphahom S, Nguyet Ly M, Soum V, Pyun N, Kwon OS, Rodthongkum N, Shin K (2020) Recent advances in Microfluidic Paper-based Analytical devices toward High-Throughput Screening. Molecules 25(13). https://doi.org/10.3390/molecules25132970
Silva-Neto HA, Arantes IVS, Ferreira AL, do Nascimento GHM, Meloni GN, de Araujo WR, Paixa TRLC, Coltro WKT (2023) Recent advances on paper-based microfluidic devices for bioanalysis. TrAC Trends Anal Chem 158. https://doi.org/10.1016/j.trac.2022.116893
Yuan M, Li C, Wang M, Cao H, Ye T, Hao L, Wu X, Yin F, Yu J, Xu F (2023) Low-cost, portable, on-site fluorescent detection of as(III) by a paper-based microfluidic device based on aptamer and smartphone imaging. Microchim Acta 190(3). https://doi.org/10.1007/s00604-023-05693-3
Nie ZH, Nijhuis CA, Gong JL, Chen X, Kumachev A, Martinez AW, Narovlyansky M, Whitesides GM (2010) Electrochemical sensing in paper-based microfluidic devices. Lab Chip 10(4):477–483. https://doi.org/10.1039/b917150a
Sitanurak J, Wangdi N, Sonsa-ard TS, Teerasong S, Amornsakchai T, Nacapricha D (2018) Simple and green method for direct quantification of hypochlorite in household bleach with membraneless gas-separation microfluidic paper-based analytical device. Talanta 187:91–98. https://doi.org/10.1016/j.talanta.2018.04.077
Song J, Fang G, Zhang Y, Deng Q, Wang S (2010) Fingerprint Analysis of Ginkgo biloba Leaves and Related Health Foods by High-Performance Liquid Chromatography/Electrospray Ionization-Mass Spectrometry. J AOAC Int 93(6):1798–1805
Du Q, Wu P, Dramou P, Chen R, He H (2019) One-step fabrication of a boric acid-functionalized lanthanide metal-organic framework as a ratiometric fluorescence probe for the selective recognition of dopamine. New J Chem 43(3):1291–1298. https://doi.org/10.1039/c8nj05318a
Yu L, Feng L, Xiong L, Li S, Xu Q, Pan X, Xiao Y (2021) Multifunctional nanoscale lanthanide metal–organic framework based ratiometric fluorescence paper microchip for visual dopamine assay. Nanoscale 13(25):11188–11196. https://doi.org/10.1039/D1NR02036F
Barbosa CDES, da Luz LL, Paz FAA, Malta OL, Rodrigues MO, Júnior SA, Ferreira RAS, Carlos LD (2017) Site-selective Eu(iii) spectroscopy of highly efficient luminescent mixed-metal pb(ii)/Eu(iii) coordination polymers. RSC Adv 7(10):6093–6101. https://doi.org/10.1039/C6RA27850G
Wang H, Wang X, Liang M, Chen G, Kong RM, Xia L, Qu F (2020) A boric acid-functionalized Lanthanide Metal-Organic Framework as a fluorescence turn-on probe for selective monitoring of Hg2+ and CH3Hg+. Anal Chem 92(4):3366–3372. https://doi.org/10.1021/acs.analchem.9b05410
Wang F, Zhang X, Huangfu C, Zhi H, Zhu M, Feng L (2023) Confinement of high-loading probes within silica film for paper-based probe with enhanced imidacloprid sensitivity application. Sens Actuators B 375:132919. https://doi.org/10.1016/j.snb.2022.132919
Tong C, Shi F, Tong X, Shi S, Ali I, Guo Y (2021) Shining natural flavonols in sensing and bioimaging. TrAC. Trends Anal Chem 137:116222. https://doi.org/10.1016/j.trac.2021.116222
Kakigi Y, Hakamatsuka T, Icho T, Goda Y, Mochizuki N (2011) Investigation of biologically active components in Ginkgo Leaf products on the Japanese Market. Biosci Biotechnol Biochem 75(4):777–779. https://doi.org/10.1271/bbb.100838
Ma YC, Mani A, Cai Y, Thomson J, Ma J, Peudru F, Chen S, Luo M, Zhang J, Chapman RG, Shi ZT (2016) An effective identification and quantification method for Ginkgo biloba flavonol glycosides with targeted evaluation of adulterated products. Phytomedicine 23(4):377–387. https://doi.org/10.1016/j.phymed.2016.02.003
Zhou X, Xiang B, Wang Z, Zhang M (2007) Determination of quercetin in extracts of Ginkgo biloba L. leaves by near-infrared reflectance spectroscopy based on interval partial least-squares (iPLS) model. Anal Lett 40(16–18):3383. https://doi.org/10.1080/00032710701689081
Acknowledgements
This research acknowledges the financial support of the Bureau of the International Cooperation of the NSFC and the Foreign Experts Department of the Ministry of Science and Technology of the People’s Republic of China through a Grant for Foreign Youth Project [No. QNJ20200010003]; the National Natural Science Foundation of China (NSFC) through the Grant [No. 81950410634]; China Pharmaceutical University 2019 high-level talent introduction research start-up fund [No. 3150050051].
Funding
http://dx.doi.org/10.13039/501100001809 National Natural Science Foundation of China 81950410634 Dr.Pierre DRAMOU 0000-0002-5606-034X the Bureau of the International Cooperation of the NSFC and the Foreign Experts Department of the Ministry of Science and Technology of the People’s Republic of China through a Grant for Foreign Youth Project QNJ20200010003 Dr. Pierre DRAMOU 0000-0002-5606-034X China Pharmaceutical University 2019 high-level talent introduction research start-up fund 3150050051 Dr. Pierre DRAMOU 0000-0002-5606-034X
Author information
Authors and Affiliations
Contributions
JG and DW: Conceptualization, method, formal analysis, investigation, writing - original draft. YC: Validation & investigation. GIU, XT, ZL, YW: Writing, review & data analysis. HH: Method, resources & supervision. DX: Review & supervision. PD: Funding acquisition, supervision, data validation, writing-review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, J., Wang, D., Chen, Y. et al. Portable paper-based probe for on-site ratiometric fluorescence determination of total flavonol glycosides in plant extract using smartphone imaging. Microchim Acta 191, 70 (2024). https://doi.org/10.1007/s00604-023-06166-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-06166-3