Abstract
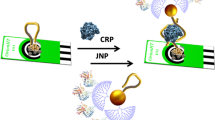
An ultra-efficient biocatalytic peroxidase-like Au-based single-atom nanozyme (Au-SAzymes) has been synthesized from isolated Au atoms on black nitrogen doped carbon (Au–N-C) using a simple complexation-adsorption-pyrolysis method. The atomic structure of AuN4 centers in black carbon was revealed by combined high-resolution transmission electron microscopy/high-angle annular dark-field scanning transmission electron microscopy. The Au-SAzymes showed a remarkable peroxidase activity with 1.7 nM as Michaelis–Menten constant, higher than most previously reported SAzyme activity. Density functional theory and Monte Carlo calculations revealed the adsorption of H2O2 on AuN4 with formation of OH* and O*. Molecular recognition was greatly enhanced via label-free integration of thiol-terminal aptamers on the surface of single Au atoms (Aptamer/Au-SAzyme) to design off–on ultrasensitive aptananozyme-based sensor for detecting thrombin and CRP with 550 pM and 500 pg mL−1 limits of detection, respectively. The Aptamer/Au-SAzyme showed satisfactory accuracy and precision when applied to the serum and plasma of COVID-19 patients. Due to the maximum Au atom utilization, approximately 3636 samples can be run per 1 mg of gold, highlighting the commercialization potential of the developed Aptamer/Au-SAzyme approach.
Graphical abstract








Similar content being viewed by others
References
Chang B, Zhang L, Wu S, Sun Z, Cheng Z (2022) Engineering single-atom catalysts toward biomedical applications. Chem Soc Rev 51:3688
Jiao L et al (2020) When nanozymes meet single-atom catalysis. Angew Chem – Int Ed 59:2565–2576
Jiao L et al (2021) Single-atom catalysts boost signal amplification for biosensing. Chem Soc Rev 50:750–765
Wu Y et al (2020) Cascade reaction system integrating single-atom nanozymes with abundant Cu sites for enhanced biosensing. Anal Chem 92:3373–3379
Zhang T, Walsh AG, Yu J, Zhang P (2021) Single-atom alloy catalysts: structural analysis, electronic properties and catalytic activities. Chem Soc Rev 50:569–588
Lu X, Gao S, Lin H, Shi J (2021) Single-atom catalysts for nanocatalytic tumor therapy. Small 17:2004467
Xiang H, Feng W, Chen Y (2020) Single-atom catalysts in catalytic biomedicine. Adv Mater 32:1905994
Su R, Zhang H, Chen F, Wang Z, Huang L (2022) Applications of single atom catalysts for environmental management. Int J Environ Res Public Health 19:11155
Wang H, Wan K, Shi X (2019) Recent advances in nanozyme research. Adv Mater 31:1–10
Li Z et al (2018) Peroxidase-mimicking nanozyme with enhanced activity and high stability based on metal–support interactions. Chem–A Eur J 24:409–415
Thangudu S, Su C-H (2021) Peroxidase mimetic nanozymes in cancer phototherapy: Progress and perspectives. Biomolecules 11:1015
Yan X, Gao L (2020) Nanozymology: An Overview. In: Yan X (eds) Nanozymology. Nanostructure Science and Technology. Springer, Singapore. https://doi.org/10.1007/978-981-15-1490-6_1
Pei J et al (2020) Single-atom nanozymes for biological applications. Biomater Sci 8:6428–6441
Unnikrishnan B, Lien C-W, Chu H-W, Huang C-C (2021) A review on metal nanozyme-based sensing of heavy metal ions: Challenges and future perspectives. J Hazard Mater 401:123397
Huang L, Chen J, Gan L, Wang J, Dong S (2019) Single-atom nanozymes. Sci Adv 5:eaav5490
Jiao L et al (2019) Fe-N-C Single-atom nanozymes for the intracellular hydrogen peroxide detection. Anal Chem 91:11994–11999
Ding H, Hu B, Zhang B et al (2021) Carbon-based nanozymes for biomedical applications. Nano Res 14:570–583. https://doi.org/10.1007/s12274-020-3053-9
Zhang R, Yan X, Fan K (2021) Nanozymes inspired by natural enzymes. Acc Mater Res 2:534–547
Yang X-F et al (2013) Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc Chem Res 46:1740–1748
Samantaray MK et al (2019) The comparison between single atom catalysis and surface organometallic catalysis. Chem Rev 120:734–813
Zhang H, Liu G, Shi L, Ye J (2018) Single-atom catalysts: emerging multifunctional materials in heterogeneous catalysis. Adv Energy Mater 8:1701343
Xu H, Zhao Y, Wang Q, He G, Chen H (2022) Supports promote single-atom catalysts toward advanced electrocatalysis. Coord Chem Rev 451:214261
Kaiser SK, Chen Z, Faust Akl D, Mitchell S, Pérez-Ramírez J (2020) Single-atom catalysts across the periodic table. Chem Rev 120:11703–11809
Zhang J et al (2018) Single-atom Au/NiFe layered double hydroxide electrocatalyst: probing the origin of activity for oxygen evolution reaction. J Am Chem Soc 140:3876–3879
Li J et al (2018) Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat Catal 1:935–945
Wu W, Huang L, Wang E, Dong S (2020) Atomic engineering of single-atom nanozymes for enzyme-like catalysis. Chem Sci 11:9741–9756
Qiao B et al (2011) Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat Chem 3:634–641
Chen W et al (2017) Rational design of single molybdenum atoms anchored on N-doped carbon for effective hydrogen evolution reaction. Angew Chem Int Ed 56:16086–16090
Zhao C et al (2017) Ionic exchange of metal–organic frameworks to access single nickel sites for efficient electroreduction of CO2. J Am Chem Soc 139:8078–8081
Martinelli LM, Carucci A, Payano VJH et al (2023) Translational comparison of the human and mouse yolk sac development and function. Reprod Sci 30:41–53. https://doi.org/10.1007/s43032-022-00872-8
Jiao L et al (2019) Fe–N–C single-atom nanozymes for the intracellular hydrogen peroxide detection. Anal Chem 91:11994–11999
Pokhrel S, Chhetri R (2021) A literature review on impact of COVID-19 pandemic on teaching and learning. Higher Education Future 8:133–141
Castagnoli R et al (2020) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr 174:882–889
Tan HW, Xu Y, Lau ATY (2020) Angiotensin-converting enzyme 2: the old door for new severe acute respiratory syndrome coronavirus 2 infection. Rev Med Virol 30:e2122
Ganatra S et al (2020) Management of cardiovascular disease during coronavirus disease (COVID-19) pandemic. Trends Cardiovasc Med 30(6):315–325. https://doi.org/10.1016/j.tcm.2020.05.004
Que Y et al (2022) Cytokine release syndrome in COVID-19: a major mechanism of morbidity and mortality. Int Rev Immunol 41:217–230
Hu B, Huang S, Yin L (2021) The cytokine storm and COVID-19. J Med Virol 93:250–256
Patel S, Saxena B, Mehta P (2021) Recent updates in the clinical trials of therapeutic monoclonal antibodies targeting cytokine storm for the management of COVID-19. Heliyon 7:e06158
Kaushal K et al (2022) Serum ferritin as a predictive biomarker in COVID-19. A systematic review, meta-analysis and meta-regression analysis. J Crit Care 67:172–181
Mosquera-Sulbaran JA, Pedreañez A, Carrero Y, Callejas D (2021) C-reactive protein as an effector molecule in Covid-19 pathogenesis. Rev Med Virol 31:e2221
Chaudhary R et al (2021) Thromboinflammatory biomarkers in COVID-19: systematic review and meta-analysis of 17,052 patients. Mayo Clin Proc: Innov, Qual Outcomes 5:388–402
Iwamura APD et al (2021) Immunity and inflammatory biomarkers in COVID-19: a systematic review. Rev Med Virol 31:e2199
Chung MK et al (2021) COVID-19 and cardiovascular disease: from bench to bedside. Circ Res 128:1214–1236
Talasaz AH, Kakavand H, Van Tassell B et al (2021) Cardiovascular Complications of COVID-19: Pharmacotherapy Perspective. Cardiovasc Drugs Ther 35:249–259. https://doi.org/10.1007/s10557-020-07037-2
Korompoki E et al (2022) Late-onset hematological complications post COVID-19: an emerging medical problem for the hematologist. Am J Hematol 97:119–128
Papakonstantinou E et al (2021) Haematological malignancies implications during the times of the COVID-19 pandemic. Oncol Lett 22:1–7
Mungmunpuntipantip R, Wiwanitkit V (2022) COVID-19, neurovascular thrombotic problem and short summary on blood coagulation disorder: a brief review. Egypt J Neurol, Psychiatr Neurosurg 58:1–5
Aliter KF, Al-Horani RA (2021) Thrombin inhibition by argatroban: potential therapeutic benefits in COVID-19. Cardiovasc Drugs Ther 35:195–203
Sriram K, Insel PA (2021) Inflammation and thrombosis in COVID-19 pathophysiology: proteinase-activated and purinergic receptors as drivers and candidate therapeutic targets. Physiol Rev 101:545–567
Ali GK, Omer KM (2022) Nanozyme and stimulated fluorescent Cu-based metal–organic frameworks (Cu-MOFs) functionalized with engineered aptamers as a molecular recognition element for thrombin detection in the plasma of COVID-19 patients. ACS Omega 7:36804–36810
Di Cera E (2008) Thrombin. Mol Aspects Med 29:203–254
Mann KG (2003) Thrombin formation. Chest 124:4S-10S
Hurlimann J, Thorbecke GJ, Hochwald GM (1966) The liver as the site of C-reactive protein formation. J Exp Med 123:365–378
Eklund CM (2009) Proinflammatory cytokines in CRP baseline regulation. Adv Clin Chem 48:111–136
Meurman J, Celec P, Gutierrez AM, Sjöwall C (2022) Pronounced diurnal pattern of salivary C-reactive protein (CRP) with modest associations. Diagn Ther Appl Pentraxin Pentraxin-Assoc Proteins
Eisenhardt SU, Thiele JR, Bannasch H, Stark GB, Peter K (2009) C-reactive protein: how conformational changes influence inflammatory properties. Cell Cycle 8:3885–3892
Agrawal A (2005) CRP after 2004. Mol Immunol 42:927–930
Ali GK, Omer KM (2022) Ultrasensitive aptamer-functionalized Cu-MOF fluorescent nanozyme as an optical biosensor for detection of C-reactive protein. Anal Biochem 114928. https://doi.org/10.1016/j.ab.2022.114928
Kotru S et al (2021) Electrochemical sensing: A prognostic tool in the fight against COVID-19. TrAC, Trends Anal Chem 136:116198
Thuerlemann C, Haeberli A, Alberio L (2009) Monitoring thrombin generation by electrochemistry: development of an amperometric biosensor screening test for plasma and whole blood. Clin Chem 55:505–512
Mahmoud M, Ruppert C, Rentschler S, Laufer S, Deigner H-P (2021) Combining aptamers and antibodies: lateral flow quantification for thrombin and interleukin-6 with smartphone readout. Sens Actuators, B Chem 333:129246
Nagy-Simon T, Hada A-M, Suarasan S, Potara M (2021) Recent advances on the development of plasmon-assisted biosensors for detection of C-reactive protein. J Mol Struct 1246:131178
Mallakpour S, Azadi E, Hussain CM (2021) Fight against COVID-19 pandemic with the help of carbon-based nanomaterials. New J Chem 45:8832–8846
Maddali H, Miles CE, Kohn J, O’Carroll DM (2021) Optical biosensors for virus detection: prospects for SARS-CoV-2/COVID-19. ChemBioChem 22:1176–1189
Sh. Mohammed Ameen S, Sher Mohammed NM, Omer KM (2022) Visual monitoring of silver ions and cysteine using bi-ligand Eu-based metal organic framework as a reference signal: Color tonality. Microchem J 181:107721
Mohammed Ameen SS, Sher Mohammed NM, Omer KM (2023) Ultra-small highly fluorescent zinc-based metal organic framework nanodots for ratiometric visual sensing of tetracycline based on aggregation induced emission. Talanta 254:124178
Mohammed Ameen SS, Qasim FO, Alhasan HS, Hama Aziz KH, Omer KM (2023) Intrinsic dual-state emission zinc-based MOF rodlike nanostructures with applications in smartphone readout visual-based detection for tetracycline: MOF-Based Color Tonality. ACS Appl Mater Interfaces 15:46098–46107
Wang C, Sun Y, Zhao Q (2020) A sensitive thrombin-linked sandwich immunoassay for protein targets using high affinity phosphorodithioate modified aptamer for thrombin labeling. Talanta 207:120280
Kurantsin-Mills J, Ofosu FA, Safa TK, Siegel RS, Lessin LS (1992) Plasma factor VII and thrombin–antithrombin III levels indicate increased tissue factor activity in sickle cell patients. Br J Haematol 81:539–544
Kushner I, Somerville JA (1970) Estimation of the molecular size of C-reactive protein and Cx-reactive protein in serum. Biochim Biophys Acta (BBA)-Protein Struct 207:105–114
Algarra M, Gomes D, da Silva JCGE (2013) Current analytical strategies for C-reactive protein quantification in blood. Clin Chim Acta 415:1–9
White D et al (2021) Evaluation of COVID-19 coagulopathy; laboratory characterization using thrombin generation and nonconventional haemostasis assays. Int J Lab Hematol 43:123–130
Ong DSY, de Man SJ, Lindeboom FA, Koeleman JGM (2020) Comparison of diagnostic accuracies of rapid serological tests and ELISA to molecular diagnostics in patients with suspected coronavirus disease 2019 presenting to the hospital. Clin Microbiol Infect 26:1094-e7
Adachi T, Nakamura Y (2019) Aptamers: A review of their chemical properties and modifications for therapeutic application. Molecules 24:4229
Mascini M (2009) Aptamers in bioanalysis. Wiley
Kong HY, Byun J (2013) Nucleic acid aptamers: new methods for selection, stabilization, and application in biomedical science. Biomol Ther 21:423
Ospina-Villa JD et al (2018) Advances on aptamers against protozoan parasites. Genes 9:584
Cho EJ, Lee J-W, Ellington AD (2009) Applications of aptamers as sensors. Annu Rev Anal Chem 2:241–264
Zhang Y, Lai BS, Juhas M (2019) Recent advances in aptamer discovery and applications. Molecules 24:941
Ali GK, Omer KM (2022) Molecular imprinted polymer combined with aptamer (MIP-aptamer) as a hybrid dual recognition element for bio(chemical) sensing applications. Rev Talanta 236:122878
Hong P, Li W, Li J (2012) Applications of aptasensors in clinical diagnostics. Sensors 12:1181–1193
Yang H et al (2019) A universal ligand mediated method for large scale synthesis of transition metal single atom catalysts. Nat Commun 10:1–9
Lee AY et al (2021) Raman study of D* band in graphene oxide and its correlation with reduction. Appl Surf Sci 536:147990
Brown SDM, Jorio A, Dresselhaus MS, Dresselhaus G (2001) Observations of the D-band feature in the Raman spectra of carbon nanotubes. Phys Rev B 64:73403
Liu J et al (2017) Palladium–gold single atom alloy catalysts for liquid phase selective hydrogenation of 1-hexyne. Catal Sci Technol 7:4276–4284
Carter JH et al (2019) Enhanced activity and stability of gold/ceria-titania for the low-temperature water-gas shift reaction. Front Chem 7:443
Hellgren N, Haasch RT, Schmidt S, Hultman L, Petrov I (2016) Interpretation of X-ray photoelectron spectra of carbon-nitride thin films: new insights from in situ XPS. Carbon 108:242–252
Vilian ATE et al (2020) Improved conductivity of flower-like MnWO4 on defect engineered graphitic carbon nitride as an efficient electrocatalyst for ultrasensitive sensing of chloramphenicol. J Hazard Mater 399:122868
Luo Z et al (2011) Pyridinic N doped graphene: synthesis, electronic structure, and electrocatalytic property. J Mater Chem 21:8038–8044
Wang Y et al (2021) Tautomeric molecule acts as a “sunscreen” for metal halide perovskite solar cells. Angew Chem 133:8755–8759
Viljas JK, Cuevas JC (2007) Role of electronic structure in photoassisted transport through atomic-sized contacts. Phys Rev B 75:75406
Biggs S, Mulvaney P (1994) Measurement of the forces between gold surfaces in water by atomic force microscopy. J Chem Phys 100:8501–8505
Schnell S (2014) Validity of the Michaelis-Menten equation - steady-state or reactant stationary assumption: That is the question. FEBS J 281:464–472
Rodriguez J-MG, Hux NP, Philips SJ, Towns MH (2019) Michaelis-Menten graphs, lineweaver–Burk plots, and reaction schemes: investigating introductory biochemistry students’ conceptions of representations in enzyme kinetics. J Chem Educ 96:1833–1845
Ji S et al (2021) Matching the kinetics of natural enzymes with a single-atom iron nanozyme. Nat Catal 4:407–417
Gao L et al (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2:577–583
Jiao L et al (2020) Densely isolated FeN4 sites for peroxidase mimicking. ACS Catal 10:6422–6429
Shao B et al (2022) Mn-doped single atom nanozyme composited Au for enhancing enzymatic and photothermal therapy. J Colloid Interface Sci 628:419–434
Lin Z et al (2020) A facile route for constructing Cu–N–C peroxidase mimics. J Mater Chem B 8:8599–8606
Chen Y et al (2021) Thermal atomization of platinum nanoparticles into single atoms: an effective strategy for engineering high-performance nanozymes. J Am Chem Soc 143:18643–18651
Chen Y et al (2018) Enhanced oxygen reduction with single-atomic-site iron catalysts for a zinc-air battery and hydrogen-air fuel cell. Nat Commun 9:5422
Deng D et al (2015) A single iron site confined in a graphene matrix for the catalytic oxidation of benzene at room temperature. Sci Adv 1:e1500462
Xu B et al (2022) A bioinspired five-coordinated single-atom iron nanozyme for tumor catalytic therapy. Adv Mater 34:2107088
Xu B et al (2019) A single-atom nanozyme for wound disinfection applications. Angew Chem 131:4965–4970
Acknowledgements
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-RG23097).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ali, G.K., Algethami, F.K. & Omer, K.M. Gold single atom-based aptananozyme as an ultrasensitive and selective colorimetric probe for detection of thrombin and C-reactive protein. Microchim Acta 191, 59 (2024). https://doi.org/10.1007/s00604-023-06147-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-06147-6