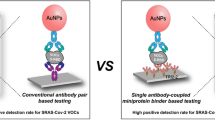
Abstract
A lateral flow assay (LFA) strip based on dual 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB)–encoded satellite Fe3O4@Au (Mag@Au) SERS tags with nanogap is reported for ultrasensitive and simultaneous diagnosis of two SARS-CoV-2 functional proteins. Composed of Fe3O4 core, satellite gold shell with nanogaps, and double-layer DTNB, the Mag@Au nanoparticles with an average size of 238 nm were designed as multifunctional tags to efficiently enrich the target SARS-CoV-2 protein from complex samples, significantly enhancing the SERS signal of the LFA strip and provide quantitative SERS detection of analyte on test lines. The developed dual DTNB-encoded satellite Mag@Au-based LFA allowed simultaneous quantification of spike (S) protein and nucleocapsid (NP) protein with detection limits of 23 pg mL−1 and 2 pg mL−1, respectively, lower than commercial ELISA kits and reported SERS-LFA detection system–based Au NPs and Fe3O4@3 nm Au MNPs. This magnetic SERS-LFA also showed high performance of multi-variant strain detection and further distinguished clinical samples of Omicron variant infection, demonstrating the potential of in situ detection of respiratory virus diseases.
Graphical Abstract







Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available on request.
References
Lavine JS, Bjornstad ON, Antia R (2021) Immunological characteristics govern the transition of COVID-19 to endemicity. Science 371:741–745. https://doi.org/10.1126/science.abe6522
Carabelli AM, Peacock TP, Thorne LG et al (2023) SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat Rev Microbiol 21:162–177. https://doi.org/10.1038/s41579-022-00841-7
Alizadehsani R, Alizadeh Sani Z, Behjati M et al (2021) Risk factors prediction, clinical outcomes, and mortality in COVID-19 patients. J Med Virol 93:2307–2320. https://doi.org/10.1002/jmv.26699
Wang C, Yang X, Zheng S, Cheng X, Xiao R, Li Q, Wang W, Liu X, Wang S (2021) Development of an ultrasensitive fluorescent immunochromatographic assay based on multilayer quantum dot nanobead for simultaneous detection of SARS-CoV-2 antigen and influenza A virus. Sens Actuators B Chem 345:130372. https://doi.org/10.1016/j.snb.2021.130372
Ganesh B, Rajakumar T, Malathi M, Manikandan N, Nagaraj J, Santhakumar A, Elangovan A, Malik YS (2021) Epidemiology and pathobiology of SARS-CoV-2 (COVID-19) in comparison with SARS, MERS: an updated overview of current knowledge and future perspectives. Clin Epidemiol Glob Health 10:100694. https://doi.org/10.1016/j.cegh.2020.100694
Yakoh A, Pimpitak U, Rengpipat S, Hirankarn N, Chailapakul O, Chaiyo S (2021) Paper-based electrochemical biosensor for diagnosing COVID-19: detection of SARS-CoV-2 antibodies and antigen. Biosens Bioelectron 176:112912. https://doi.org/10.1016/j.bios.2020.112912
Wolfel R, Corman VM, Guggemos W et al (2020) Virological assessment of hospitalized patients with COVID-2019. Nature 581:465–469. https://doi.org/10.1038/s41586-020-2196-x
Brandmeier JC, Jurga N, Grzyb T, Hlavacek A, Oborilova R, Skladal P, Farka Z, Gorris HH (2023) Digital and analog detection of SARS-CoV-2 nucleocapsid protein via an upconversion-linked immunosorbent assay. Anal Chem 95:4753–4759. https://doi.org/10.1021/acs.analchem.2c05670
Yang Y, Li H, Jones L et al (2023) Rapid detection of SARS-CoV-2 RNA in human nasopharyngeal specimens using surface-enhanced raman spectroscopy and deep learning algorithms. ACS Sens 8:297–307. https://doi.org/10.1021/acssensors.2c02194
Novodchuk I, Kayaharman M, Prassas I et al (2022) Electronic field effect detection of SARS-CoV-2 N-protein before the onset of symptoms. Biosens Bioelectron 210:114331. https://doi.org/10.1016/j.bios.2022.114331
Corman VM, Haage VC, Bleicker T et al (2021) Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: a single-centre laboratory evaluation study. Lancet Microbe 2:e311–e319. https://doi.org/10.1016/S2666-5247(21)00056-2
Peeling RW, Heymann DL, Teo YY, Garcia PJ (2022) Diagnostics for COVID-19: moving from pandemic response to control. Lancet 399:757–768. https://doi.org/10.1016/S0140-6736(21)02346-1
Vandenberg O, Martiny D, Rochas O, van Belkum A, Kozlakidis Z (2021) Considerations for diagnostic COVID-19 tests. Nat Rev Microbiol 19:171–183. https://doi.org/10.1038/s41579-020-00461-z
Zhou Y, Zhang L, Xie Y, Wu J (2022) Advancements in detection of SARS-CoV-2 infection for confronting COVID-19 pandemics. Lab Invest 102:4–13. https://doi.org/10.1038/s41374-021-00663-w
Gao J, Quan L (2020) Current status of diagnostic testing for SARS-CoV-2 infection and future developments: a review. Med Sci Monit 26:e928552. https://doi.org/10.12659/MSM.928552
Yang X, Yu Q, Cheng X, Wei H, Zhang X, Rong Z, Wang C, Wang S (2023) Introduction of multilayered dual-signal nanotags into a colorimetric-fluorescent coenhanced immunochromatographic assay for ultrasensitive and flexible monitoring of SARS-CoV-2. ACS Appl Mater Interfaces 15:12327–12338. https://doi.org/10.1021/acsami.2c21042
SomboracBacura A, Dorotic M, Grosic L, Dzimbeg M, Dodig S (2021) Current status of the lateral flow immunoassay for the detection of SARS-CoV-2 in nasopharyngeal swabs. Biochem Med (Zagreb) 31:020601. https://doi.org/10.11613/BM.2021.020601
Zhang P, Chen L, Hu J et al (2022) Magnetofluidic immuno-PCR for point-of-care COVID-19 serological testing. Biosens Bioelectron 195:113656. https://doi.org/10.1016/j.bios.2021.113656
Mahshid SS, Flynn SE, Mahshid S (2021) The potential application of electrochemical biosensors in the COVID-19 pandemic: a perspective on the rapid diagnostics of SARS-CoV-2. Biosens Bioelectron 176:112905. https://doi.org/10.1016/j.bios.2020.112905
Kim S, Hao Y, Miller EA et al (2021) Vertical flow cellulose-based assays for SARS-CoV-2 antibody detection in human serum. ACS Sens 6:1891–1898. https://doi.org/10.1021/acssensors.1c00235
Park Y, Ryu B, Ki SJ, Chen M, Liang X, Kurabayashi K (2023) Bioinspired plasmo-virus for point-of-care SARS-CoV-2 detection. Nano Lett 23:98–106. https://doi.org/10.1021/acs.nanolett.2c03700
Duan X, Shi Y, Zhang X et al (2022) Dual-detection fluorescent immunochromatographic assay for quantitative detection of SARS-CoV-2 spike RBD-ACE2 blocking neutralizing antibody. Biosens Bioelectron 199:113883. https://doi.org/10.1016/j.bios.2021.113883
Yang LF, Kacherovsky N, Panpradist N, Wan R, Liang J, Zhang B, Salipante SJ, Lutz BR, Pun SH (2022) Aptamer sandwich lateral flow assay (AptaFlow) for antibody-free SARS-CoV-2 detection. Anal Chem 94:7278–7285. https://doi.org/10.1021/acs.analchem.2c00554
Silvestri A, Zayas-Arrabal J, Vera-Hidalgo M et al (2023) Ultrasensitive detection of SARS-CoV-2 spike protein by graphene field-effect transistors. Nanoscale 15:1076–1085. https://doi.org/10.1039/d2nr05103f
Langer J, Jimenez de Aberasturi D, Aizpurua J et al (2020) Present and future of surface-enhanced raman scattering. ACS Nano 14:28–117. https://doi.org/10.1021/acsnano.9b04224
Lee SH, Hwang J, Kim K et al (2019) Quantitative serodiagnosis of scrub typhus using surface-enhanced raman scattering-based lateral flow assay platforms. Anal Chem 91:12275–12282. https://doi.org/10.1021/acs.analchem.9b02363
Khlebtsov B and Khlebtsov N (2020) Surface-enhanced raman scattering-based lateral-flow immunoassay. Nanomaterials (Basel) 10. https://doi.org/10.3390/nano10112228
Zhang D, Huang L, Liu B, Ni H, Sun L, Su E, Chen H, Gu Z, Zhao X (2018) Quantitative and ultrasensitive detection of multiplex cardiac biomarkers in lateral flow assay with core-shell SERS nanotags. Biosens Bioelectron 106:204–211. https://doi.org/10.1016/j.bios.2018.01.062
Wang C, Wang C, Li J, Tu Z, Gu B, Wang S (2022) Ultrasensitive and multiplex detection of four pathogenic bacteria on a bi-channel lateral flow immunoassay strip with three-dimensional membrane-like SERS nanostickers. Biosens Bioelectron 214:114525. https://doi.org/10.1016/j.bios.2022.114525
Moraes Silva S, Tavallaie R, Sandiford L, Tilley RD, Gooding JJ (2016) Gold coated magnetic nanoparticles: from preparation to surface modification for analytical and biomedical applications. Chem Commun (Camb) 52:7528–7540. https://doi.org/10.1039/c6cc03225g
Schaumburg F, Carrell CS, Henry CS (2019) Rapid bacteria detection at low concentrations using sequential immunomagnetic separation and paper-based isotachophoresis. Anal Chem 91:9623–9630. https://doi.org/10.1021/acs.analchem.9b01002
Wang C, Wang C, Wang X, Wang K, Zhu Y, Rong Z, Wang W, Xiao R, Wang S (2019) Magnetic SERS strip for sensitive and simultaneous detection of respiratory viruses. ACS Appl Mater Interfaces 11:19495–19505. https://doi.org/10.1021/acsami.9b03920
Tu J, Wu T, Yu Q, Li J, Zheng S, Qi K, Sun G, Xiao R, Wang C (2023) Introduction of multilayered magnetic core-dual shell SERS tags into lateral flow immunoassay: a highly stable and sensitive method for the simultaneous detection of multiple veterinary drugs in complex samples. J Hazard Mater 448:130912. https://doi.org/10.1016/j.jhazmat.2023.130912
Liu Z, Wang C, Zheng S, Yang X, Han H, Dai Y, Xiao R (2023) Simultaneously ultrasensitive and quantitative detection of influenza A virus, SARS-CoV-2, and respiratory syncytial virus via multichannel magnetic SERS-based lateral flow immunoassay. Nanomedicine 47:102624. https://doi.org/10.1016/j.nano.2022.102624
McMahon JM, Li S, Ausman LK, Schatz GC (2011) Modeling the effect of small gaps in surface-enhanced raman spectroscopy. The J Phys Chem C 116:1627–1637. https://doi.org/10.1021/jp207661y
Ding S, Yi J, Li J, Ren B, Wu D, Panneerselvam R, and Tian Z (2016) Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nature Reviews Materials 1. https://doi.org/10.1038/natrevmats.2016.21
Shen W, Wang C, Zheng S, Jiang B, Li J, Pang Y, Wang C, Hao R, Xiao R (2022) Ultrasensitive multichannel immunochromatographic assay for rapid detection of foodborne bacteria based on two-dimensional film-like SERS labels. J Hazard Mater 437:129347. https://doi.org/10.1016/j.jhazmat.2022.129347
Liu X, Yang X, Li K, Liu H, Xiao R, Wang W, Wang C, and Wang S (2020) Fe3O4@Au SERS tags-based lateral flow assay for simultaneous detection of serum amyloid A and C-reactive protein in unprocessed blood sample. Sensors and Actuators B: Chemical 320. https://doi.org/10.1016/j.snb.2020.128350
Funding
This study was supported by the National Key Research and Development Program of China (Grant nos. 2021YFC2301102).
Author information
Authors and Affiliations
Contributions
RX, GHW, and CWW designed and managed the project. XXL and XSY performed all the experiments. QQL and YLD did the analysis of PCR. SPX provided clinical samples. XXL and XSY wrote the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Yang, X., Wang, C. et al. A nanogap-enhanced SERS nanotag–based lateral flow assay for ultrasensitive and simultaneous monitoring of SARS-CoV-2 S and NP antigens. Microchim Acta 191, 104 (2024). https://doi.org/10.1007/s00604-023-06126-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-06126-x