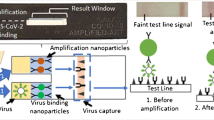
Abstract
A highly sensitive colorimetric method (glycan-based nano(e)zyme) was developed for sensitive and rapid detection of the SARS-CoV-2 virus based on N-acetyl neuraminic acid (sialic acid)-functionalized gold nanoparticles (SA-Au NZs). A number of techniques were used to characterize the prepared nanomaterials including XRD, FT-IR, UV–vis, DLS, and TEM. DLS analysis indicates an average hydrodynamic size of 34 nm, whereas TEM analysis indicates an average particle size of 15.78 nm. This observation confirms that water interacts with nanoparticle surfaces, resulting in a large hydrodynamic diameter. The peroxidase-like activity of SA-Au NZs was examined with SARS-CoV-2 and influenza viruses (influenza A (H1N1), influenza A (H3N2), and influenza B). UV–visible spectroscopy was used to monitor and record the results, as well as naked eye detection (photographs). SA-Au NZs exhibit a change in color from light red to purple when SARS-CoV-2 is present, and they exhibit a redshift in their spectrum. N-acetyl neuraminic acid interacts with SARS-CoV-2 spike glycoprotein, confirming its ability to bind glycans. As a result, SA-Au NZs can detect COVID-19 with sensitivity and specificity of over 95% and 98%, respectively. This method was approved by testing saliva samples from 533 suspected individuals at Ghaem Hospital of Mashhad, Mashhad, Iran. Sensitivity and specificity were calculated by comparing the results with the definitive results. The positive results were accompanied by a color change from bright red to purple within five minutes. Statistical analysis was performed based on variables such as age, gender, smoking, diabetes, hypertension, and lung involvement. In clinical trials, it was demonstrated that this method can be used to diagnose SARS-CoV-2 in a variety of places, such as medical centers, hospitals, airports, universities, and schools.
Graphical Abstract








Similar content being viewed by others
References
Peiris JSM, Lai ST, Poon LLM, Guan Y, Yam LYC, Lim W (2003) SARS Study Group Coronavirus as a possible cause of severe acute respiratory syndrome. The Lancet 361(9366):1319–1325
Caruana G, Croxatto A, Coste AT, Opota O, Lamoth F, Jaton K, Greub G (2020) Diagnostic strategies for SARS-CoV-2 infection and interpretation of microbiological results. Clin Microbiol Infect 26(9):1178–1182
Kubina R, Dziedzic A (2020) Molecular and serological tests for COVID-19. A comparative review of SARS-CoV-2 coronavirus laboratory and point-of-care diagnostics. Diagnostics 10(6):434
CDC.Overview of Testing for SARS-CoV-2. (2021). https://www.cdc.gov/coronavirus2019ncov/hcp/testingoverview.html
Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Drosten C (2020) Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 25(3):2000045
Chen W, Lan Y, Yuan X, Deng X, Li Y, Cai X, Tang X (2020) Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect 9(1):469–473
Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, Ji W (2020) Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology 296:E115–E117
Dhar BC (2022) Diagnostic assay and technology advancement for detecting SARS-CoV-2 infections causing the COVID-19 pandemic. Anal Bioanal Chem 414(9):2903–2934
Ezennia IJ, Nduka SO, Ekwunife OI (2017) Cost benefit analysis of malaria rapid diagnostic test: the perspective of Nigerian community pharmacists. Malar J 16(1):1–10
KantiáNandi C (2016) Effect of surface chemistry and morphology of gold nanoparticle on the structure and activity of common blood proteins. New J Chem 40(6):4879–4883
Li M, Cushing SK, Wu N (2015) Plasmon-enhanced optical sensors: a review. Analyst 140(2):386–406
Abdelrahman Z, Li M, Wang X (2020) Comparative review of SARS-CoV-2, SARS-CoV, MERS-CoV, and influenza a respiratory viruse. Front Immunol 11:2309. https://doi.org/10.3389/fimmu.2020.552909
Li W, Hulswit RJ, Widjaja I, Raj VS, McBride R, Peng W, Bosch BJ (2017) Identification of sialic acid-binding function for the Middle East respiratory syndrome coronavirus spike glycoprotein. Proc National Acad Sci 114(40):E8508–E8517
Bolles M, Donaldson E, Baric R (2011) SARS-CoV and emergent coronaviruses: viral determinants of interspecies transmission. Curr Opin Virol 1(6):624–634
Li L, Zhang L, Carmona U, Knez M (2014) Semi-artificial and bioactive ferroxidase with nanoparticles as the active sites. Chem Commun 50(59):8021–8023. https://doi.org/10.1039/C4CC03477E
Bastami TR, Dabirifar Z (2020) AuNPs@ PMo 12 nanozyme: Highly oxidase mimetic activity for sensitive and specific colorimetric detection of acetaminophen. RSC Adv 10(59):35949–35956
Lin Y, Li Z, Chen Z, Ren J, Qu X (2013) Mesoporous silica-encapsulated gold nanoparticles as artificial enzymes for self-activated cascade catalysis. Biomaterials 34(11):2600–2610
Ahmed SR, Kim J, Suzuki T, Lee J, Park EY (2016) Detection of influenza virus using peroxidase-mimic of gold nanoparticles. Biotechnol Bioeng 113(10):2298–2303
Tang L, Li J (2017) Plasmon-Based colorimetric nanosensors for ultrasensitive molecular diagnostics. ACS Sensors 2(7):857–875. https://doi.org/10.1021/acssensors.7b00282
Hafez ME, Ma H, Ma W, Long YT (2019) Unveiling the intrinsic catalytic activities of single-gold-nanoparticle-based enzyme mimetics. Angew Chem 131(19):6393–6398
Fernandes AR, Jesus J, Martins P, Figueiredo S, Rosa D, Martins LM, Baptista PV (2017) Multifunctional gold-nanoparticles: a nanovectorization tool for the targeted delivery of novel chemotherapeutic agents. J Control Release 245:52–61
Zhang Z (2017) Plasmonic catalysis: principles and applications - ACS catalysis. Springer, Singapore
Li N, Zhao P, Astruc D (2014) Gold nanoparticles: synthesis, properties, biomedical applications, and toxicity. Angew Chem Int Ed 53:1756–1789. https://doi.org/10.1002/anie.201300441
Ma T, Yang W, Liu S, Zhang H, Liang F (2017) A comparison reduction of 4-nitrophenol by gold nanospheres and gold nanostars. Catalysts 7(2):38
Liu H et al (2015) Gold nanoparticle-based nano-enzyme for the highly efficient and selective catalytic reduction of 4-nitrophenol. Nanoscale 7(31):13643–13651
Huang C, Wen T, Shi FJ, Zeng XY, Jiao YJ (2020) Rapid detection of IgM antibodies against the SARS-CoV-2 virus via colloidal gold nanoparticle-based lateral-flow assay. ACS Omega 5(21):12550–12556
Alfassam HA, Nassar MS, Almusaynid MM, Khalifah BA, Alshahrani AS, Almughem FA, Tawfik EA (2021) Development of a colorimetric tool for SARS-CoV-2 and other respiratory viruses detection using sialic acid fabricated gold nanoparticles. Pharmaceutics 13(4):502
Lee C, Gaston MA, Weiss AA, Zhang P (2013) Colorimetric viral detection based on sialic acid stabilized gold nanoparticles. Biosens Bioelectron 42:236–241
Jureka AS, Silvas JA, Basler CF (2020) Propagation, inactivation, and safety testing of SARS-CoV-2. Viruses 12(6):622
Tran DH, Sugamata R, Hirose T, Suzuki S, Noguchi Y, Sugawara A, Suzuki K (2019) Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A (H1N1) pdm09 virus infection by interfering with virus internalization process. J Antibiot 72(10):759–768
Zhang Y, Villarreal E, Li GG, Wang W, Wang H (2020) Plasmonic nanozymes: engineered gold nanoparticles exhibit tunable plasmon-enhanced peroxidase-mimicking activity. J Phys Chem Lett 11(21):9321–9328
Malassis L, Dreyfus R, Murphy RJ, Hough LA, Donnio B, Murray CB (2016) One-step green synthesis of gold and silver nanoparticles with ascorbic acid and their versatile surface post-functionalization. RSC Adv 6(39):33092–33100
Isaacs D, Flowers D, Clarke JR, Valman HB, MacNaughton MR (1983) Epidemiology of coronavirus respiratory infections. Arch Dis Child 58(7):500–503
FTIR Spectroscopy of Carbohydrates by AMGMT Sprakel, JJM van der Linden (2004) J Phys Chem B, 108: 45, 2004 17448–17455
Ito M, Ikeda K, Suzuki Y, Tanaka K, Saito M (2002) An improved fluorometric high-performance liquid chromatography method for sialic acid determination: an internal standard method and its application to sialic acid analysis of human apolipoprotein E. Anal Biochem 300(2):260–266
FTIR Spectroscopy in Pharmaceutical Analysis by RK Sharma, SK Jain (2007) J Pharm Sci 96: 10 2884–2896
Tyagi H, Kushwaha A, Kumar A, Aslam M (2011) pH-dependent synthesis of stabilized gold nanoparticles using ascorbic acid. Int J Nanosci 10(04n05):857–860
Kumler WD, Sah PP (1952) Antitubercular Agent Formed by Condensing Bis (4-Aminophenyl)-Sulfone with L-Ascorbic Acid. J Am Pharm Assoc (Sci ed) 41(8):445–450
Islam M, Matouk Z, Ouldhamadouche N, Pireaux JJ, Achour A (2023) Plasma treatment of polystyrene films—effect on wettability and surface interactions with Au Nanoparticles. Plasma 6(2):322–333
Lin Y, Ren J, Qu X (2014) Catalytically active nanomaterials: a promising candidate for artificial enzymes. Acc Chem Res 47(4):1097–1105
Zhang R, Fan K, Yan X (2020) Nanozymes: created by learning from nature. Sci China Life Sci 63(8):1183–1200
Qing E, Hantak M, Perlman S, Gallagher T (2020) Distinct roles for sialoside and protein receptors in coronavirus infection. MBio 11(1):10–1128
Hulswit RJ, Lang Y, Bakkers MJ, Li W, Li Z, Schouten A, de Groot RJ (2019) Human coronaviruses OC43 and HKU1 bind to 9-O-acetylated sialic acids via a conserved receptor-binding site in spike protein domain A. Proc National Acad Sci 116(7):2681–2690
Huang X, Dong W, Milewska A, Golda A, Qi Y, Zhu QK, Sui J (2015) Human coronavirus HKU1 spike protein uses O-acetylated sialic acid as an attachment receptor determinant and employs hemagglutinin-esterase protein as a receptor-destroying enzyme. J Virol 89(14):7202–7213
Weis W, Brown JH, Cusack S, Paulson JC, Skehel JJ, Wiley DC (1988) Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature 333(6172):426–431
Baker AN, Richards SJ, Pandey S, Guy CS, Ahmad A, Hasan M, Gibson MI (2021) Glycan-based flow-through device for the detection of SARS-CoV-2. ACS Sensors 6(10):3696–3705
Fu Z, Zeng W, Cai S, Li H, Ding J, Wang C, Yang R (2021) Porous Au@Pt nanoparticles with superior peroxidase-like activity for colorimetric detection of spike protein of SARS-CoV-2. J Colloid Interface Sci 604:113–121
Behrouzi K, Lin L (2022) Gold nanoparticle based plasmonic sensing for the detection of SARS-CoV-2 nucleocapsid proteins. Biosens Bioelectron 195:113669
Karakuş E, Erdemir E, Demirbilek N, Liv L (2021) Colorimetric and electrochemical detection of SARS-CoV-2 spike antigen with a gold nanoparticle-based biosensor. Anal Chim Acta 1182:338939
Ventura BD, Cennamo M, Minopoli A, Campanile R, Censi SB, Terracciano D, Velotta R (2020) Colorimetric test for fast detection of SARS-CoV-2 in nasal and throat swabs. ACS Sensors 5(10):3043–3048
Ali MG, Ahmad MO, Husain SN (2020) Spread of corona virus disease (COVID-19) from an outbreak to pandemic in the year 2020. Asian J Res Infect Dis 3(4):37–51
Meingast CL, Joshi PU, Turpeinen DG, Xu X, Holstein M, Feroz H, Heldt CL (2021) Physiochemical properties of enveloped viruses and arginine dictate inactivation. Biotechnol J 16(7):2000342
Muhammad A, Ameer H, Haider SA, Ali I (2021) Detection of SARS-CoV-2 using real-time polymerase chain reaction in different clinical specimens: A critical review. Allergol Immunopathol 49(1):159–164
Funding
This work has been financially supported by the Mashhad University Science Research Council (MUMS/992426). This research was approved by the Ethical Committee of the Mashhad University of Medical Sciences (IRMUMS.REC.1400.156).
Author information
Authors and Affiliations
Contributions
Tahereh Rohani Bastami designed the nano biosensor, performed the data interpretation, and supervised the research team. Mehrdad Rokni fabricated the nano biosensor and tested 533 people. Zeynab Dabirifar helped with the fabrication of nano-biosensor and tests of people. Zahra Meshkat and Hamidreza Rahimi designed the protocol for the test of people. Zahra Meshkat conducted the RT-PCR results and interpretation of the results. Saeed Zibaee provided the inactivated SARS-CoV-2. Fatemeh Fotouhi provided the influenza viruses. Mojtaba Meshkat performed the statistical analysis. Elham Serki and Mahdieh Khoshakhlagh carried out RT-PCR.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rokni, M., Rohani Bastami, T., Meshkat, Z. et al. Rapid and sensitive detection of SARS-CoV-2 virus in human saliva samples using glycan based nanozyme: a clinical study. Microchim Acta 191, 36 (2024). https://doi.org/10.1007/s00604-023-06120-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-06120-3