Abstract
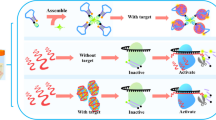
A highly structured fluorometric bioassay has been proposed for screening Staphylococcus aureus (S. aureus). The study exploits (i) the spectral attributes of the hexagonal NaYF4:Yb,Er upconversion nanoparticle (UCNP)-coated 3-aminopropyl)triethoxysilane; (ii) the intrinsic non-fluorescent quenching features of the highly stable dark blackberry (BBQ®-650) receptor; (iii) the aptamer (Apt-) biorecognition and binding affinity, and (iv) the complementary DNA hybridizer-linkage efficacy. The principle relied on the excited state energy transfer between the donor Apt-labeled NH2-UCNPs at the 3′ end, and cDNA-grafted BBQ®-650 at the 5′ end, as the effective receptors. The donor moieties in proximity (< 10.0 nm) trigger hybridization with the cDNA-grafted dark BBQ®-650, as the receptors of energy from the 2F5/2 level of Yb3+ ions to initiate the Förster resonance energy transfer pathway. This was confirmed by the decline in the excited-state lifetimes from 223.52 μs (τ1) to 179.26 μs (τ2). The existence of the target S. aureus in the bioassay attracts the Apt- resulting in the detachment of the acceptor, and disintegration of the complex configuration via conformation reversal. The re-activated fluorescence monitored at λex/em = 980/652 nm, as a function of the logarithmic concentration of S. aureus (42 to 4.2 × 108 CFU mL−1), yielded an ultra-low detection response of 2.0 CFU mL−1. The bioassay screening of S. aureus in real samples revealed satisfactory recoveries (92.44–107.82%) and validation results (p > 0.05). Hence, the comprehensive Apt-labeled NH2-UCNPs-cDNA-grafted dark BBQ®-650 bioassay offered fast and precise S. aureus screening in food and environmental settings.
Graphical abstract





Similar content being viewed by others
References
Liu Y, Wei Y, Cao Y, Zhu D, Yu Y, Guo M (2018) Ultrasensitive electrochemiluminescence detection of Staphylococcus aureus via enzyme-free branched DNA signal amplification probe. Biosens Bioelectron 117:830–837
Shahdordizadeh M, Taghdisi SM, Ansari N, Langroodi FA, Abnous K, Ramezani M (2017) Aptamer based biosensors for detection of Staphylococcus aureus. Sens Actuators B: Chem 241:619–635
Le Loir Y, Baron F, Gautier M (2003) [i] Staphylococcus aureus [/i] and food poisoning. Genet Mol Res: GMR 2:63–76
Baptista I, Rocha SM, Cunha A, Saraiva JA, Almeida A (2016) Inactivation of Staphylococcus aureus by high pressure processing: An overview. IFSET 36:128–149
Wu Z, Huang C, Dong Y, Zhao B, Chen Y (2022) Gold core@ platinum shell nanozyme-mediated magnetic relaxation switching DNA sensor for the detection of Listeria monocytogenes in chicken samples. Food Cont 137:108916
Ren L, Hong F, Chen Y (2022) Enzyme-free catalytic hairpin assembly reaction-mediated micro-orifice resistance assay for the ultrasensitive and low-cost detection of Listeria monocytogenes. Biosens Bioelectron 214:114490
Li Y, Wu L, Wang Z, Tu K, Pan L, Chen Y (2021) A magnetic relaxation DNA biosensor for rapid detection of Listeria monocytogenes using phosphatase-mediated Mn (VII)/Mn (II) conversion. Food Cont 125:107959
Pires SM, Desta BN, Mughini-Gras L, Mmbaga BT, Fayemi OE, Salvador EM, Gobena T, Majowicz SE, Hald T, Hoejskov PS (2021) Burden of foodborne diseases: Think global, act local. Curr Opin Food Sci 39:152–159
Farooq U, Ullah MW, Yang Q, Aziz A, Xu J, Zhou L, Wang S (2020) High-density phage particles immobilization in surface-modified bacterial cellulose for ultra-sensitive and selective electrochemical detection of Staphylococcus aureus. Biosens Bioelectron 157:112163
Nguyen TT-Q, Kim ER, Gu MB (2022) A new cognate aptamer pair-based sandwich-type electrochemical biosensor for sensitive detection of Staphylococcus aureus. Biosens Bioelectron 198:113835
Zhu S, Tang Y, Shi B, Zou W, Wang X, Wang C, Wu Y (2021) Oligonucleotide-mediated the oxidase-mimicking activity of Mn3O4 nanoparticles as a novel colorimetric aptasensor for ultrasensitive and selective detection of Staphylococcus aureus in food. Sens Actuators B: Chem 349:130809
Wei W, Haruna SA, Zhao, Y, Li H, Chen Q (2022) Surface-enhanced Raman scattering biosensor-based sandwich-type for facile and sensitive detection of Staphylococcus aureus. Sens Actuators B: Chem 364:131929
Choopara I, Suea-Ngam A, Teethaisong Y, Howes PD, Schmelcher M, Leelahavanichkul A, Thunyaharn S, Wongsawaeng D, DeMello AJ, Dean D (2021) Fluorometric paper-based, loop-mediated isothermal amplification devices for quantitative point-of-care detection of methicillin-resistant staphylococcus aureus (MRSA). ACS Sens 6:742–751
Ouyang Q, Yang Y, Ali S, Wang L, Li H, Chen Q (2021) Upconversion nanoparticles-based FRET system for sensitive detection of Staphylococcus aureus. Spectrochim Acta A 255:119734
Desai AS, Chauhan VM, Johnston AP, Esler T, Aylott JW (2014) Fluorescent nanosensors for intracellular measurements: synthesis, characterization, calibration, and measurement. Front Physiol 4:401
Auzel F (2004) Upconversion and anti-stokes processes with f and d ions in solids. Chem Rev 104:139–174
Hu K, Yu X, Chen J, Tang J, Wang L, Li Y, Tang C (2020) Production of characteristic volatile markers and their relation to Staphylococcus aureus growth status in pork. Meat Sci 160:107956
Wang M, Abbineni G, Clevenger A, Mao C, Xu S (2011) Upconversion nanoparticles: synthesis, surface modification and biological applications. Nanomed Nanotechnol Biol Med 7:710–729
Iliuk AB, Hu L, Tao WA (2011) Aptamer in bioanalytical applications. Anal Chem 83:4440–4452
Tombelli S, Minunni M, Mascini M (2007) Aptamers-based assays for diagnostics, environmental and food analysis. Biomol Eng 24:191–200
Liu R, Ali S, Haruna SA, Ouyang Q, Li H, Chen Q (2022) Development of a fluorescence sensing platform for specific and sensitive detection of pathogenic bacteria in food samples. Food Cont 131:108419
Ouyang Q, Wang L, Ahmad W, Yang Y, Chen Q (2021) Upconversion nanoprobes based on a horseradish peroxidase-regulated dual-mode strategy for the ultrasensitive detection of Staphylococcus aureus in Meat. J Agr Food Chem 69:9947–9956
Peltomaa R, Benito-Peña E, Gorris HH, Moreno-Bondi MC (2021) Biosensing based on upconversion nanoparticles for food quality and safety applications. Analyst 146:13–32
Jin B, Li Z, Zhao G, Ji J, Chen J, Yang Y, Xu R (2022) Upconversion fluorescence-based paper disc for multiplex point-of-care testing in water quality monitoring. Anal Chim Acta 1192:339388
Zhang B, Li H, Pan W, Chen Q, Ouyang Q, Zhao J (2017) Dual-color upconversion nanoparticles (UCNPs)-based fluorescent immunoassay probes for sensitive sensing foodborne pathogens. Food Anal Methods 10:2036–2045
Chen M, Song Y, Han L, Zhou D, Wang Y, Pan L, Tu K (2022) An ultrasensitive upconversion fluorescence aptasensor based on graphene oxide release and magnetic separation for Staphylococcus aureus detection. Food Anal Methods 15:2791–2800
Yüce M, Kurt H, Hussain B, Ow-Yang CW, Budak H (2018) Exploiting Stokes and anti-Stokes type emission profiles of aptamer-functionalized luminescent nanoprobes for multiplex sensing applications. ChemistrySelect 3:5814–5823
He H, Sun DW, Wu Z, Pu H, Wei Q (2022) On-off-on fluorescent nanosensing: Materials, detection strategies and recent food applications. Trends Food Sci Technol 119:243–256
Liu R, Zhang Y, Ali S, Haruna SA, He P, Li H, Ouyang Q, Chen Q (2021) Development of a fluorescence aptasensor for rapid and sensitive detection of Listeria monocytogenes in food. Food Cont 122:107808
Crisalli P, Kool ET (2011) Multi-path quenchers: efficient quenching of common fluorophores. Bioconj Chem 22:2345–2354
Marras SA, Kramer FR, Tyagi S (2002) Efficiencies of fluorescence resonance energy transfer and contact-mediated quenching in oligonucleotide probes. Nuc Acids Res 30:e122–e122
Peng X, Chen H, Draney DR, Volcheck W, Schutz-Geschwender A, Olive DM (2009) A nonfluorescent, broad-range quencher dye for Förster resonance energy transfer assays. Analytical Biochem 388:220–228
Chevalier A, Renard PY, Romieu A (2014) Straightforward synthesis of bioconjugatable azo dyes. Part 2: Black Hole Quencher-2 (BHQ-2) and BlackBerry Quencher 650 (BBQ-650) scaffolds. Tetrahed Lett 55:6764–6768
Cao X, Li S, Chen L, Ding H, Xu H, Huang Y, Li J, Liu N, Cao W, Zhu Y (2009) Combining use of a panel of ssDNA aptamers in the detection of Staphylococcus aureus. Nuc Acids Res 37:4621–4628
Xu Y, He P, Ahmad W, Hassan MM, Ali S, Li H, Chen Q (2022) Catalytic hairpin activated gold-magnetic/gold-core-silver-shell rapid self-assembly for ultrasensitive Staphylococcus aureus sensing via PDMS-based SERS platform. Biosens Bioelectron 209:114240
Wang F, Han Y, Lim CS, Lu Y, Wang J, Xu J, Chen H, Zhang C, Hong M, Liu X (2010) Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping. Nature 463:1061–1065
Zhao X, Wang Y, Li J, Huo B, Huang H, Bai J, Peng Y, Li S, Han D, Ren S (2021) A fluorescence aptasensor for the sensitive detection of T-2 toxin based on FRET by adjusting the surface electric potentials of UCNPs and MIL-101. Anal Chim Acta 1160:338450
Wu Z, Xu E, Jin Z, Irudayaraj J (2018) An ultrasensitive aptasensor based on fluorescent resonant energy transfer and exonuclease-assisted target recycling for patulin detection. Food Chem 249:136–142
Yang Q, Li J, Wang X, Peng H, Xiong H, Chen L (2019) Dual-emission color-controllable nanoparticle based molecular imprinting ratiometric fluorescence sensor for the visual detection of Brilliant Blue. Sens Actuators B: Chem 284:428–436
Jin B, Wang S, Lin M, Jin Y, Zhang S, Cui X, Gong Y, Li A, Xu F, Lu TJ (2017) Upconversion nanoparticles based FRET aptasensor for rapid and ultrasenstive bacteria detection. Biosens Bioelectron 90:525–533
Dong H, Sun LD, Yan CH (2015) Energy transfer in lanthanide upconversion studies for extended optical applications. Chem Soc Rev 44:1608–1634
Bezdekova J, Zemankova K, Hutarova J, Kociova S, Smerkova K, Adam V, Vaculovicova M (2020) Magnetic molecularly imprinted polymers used for selective isolation and detection of Staphylococcus aureus. Food Chem 321:126673
Hu Y, Sun Y, Gu J, Yang F, Wu S, Zhang C, Ji X, Lv H, Muyldermans S, Wang S (2021) Selection of specific nanobodies to develop an immuno-assay detecting Staphylococcus aureus in milk. Food Chem 353:129481
Liu X, Huang C, Qiu C, Wang Z, Cheng M, Zhang Y, Qiao Y, Guan Y, Feng X, Sun C (2022) Rapid and sensitive detection of Staphylococcus aureus using biolayer interferometry technology combined with phage lysin LysGH15. Biosens Bioelectron 198:113799
Chen W, Chen Z, Lai Q, Zhang Y, Long M, Liang B, Liu Z (2022) Specific and ultrasensitive detection of Staphylococcus aureus with a catechol-chitosan redox capacitor based electrochemical aptasensor. J Electroanal Chem 916:116357
Zhang Y, Tan W, Zhang Y, Mao H, Shi S, Duan L, Wang H, Yu J (2019) Ultrasensitive and selective detection of Staphylococcus aureus using a novel IgY-based colorimetric platform. Biosens Bioelectron 142:111570
Xie B, Wang ZP, Zhang R, Zhang Z, He Y (2022) A SERS aptasensor based on porous Au-NC nanoballoons for Staphylococcus aureus detection. Anal Chim Acta 1190:339175
Funding
The authors received financial support from “entrepreneurship and innovation project of Jiangsu province, China (No: JSSCBS20210930).”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmad, W., Wang, L., Zareef, M. et al. Ultrasensitive detection of Staphylococcus aureus using a non-fluorescent cDNA-grafted dark BBQ®-650 chromophore integrated hydrophilic upconversion nanoparticles/aptamer system. Microchim Acta 190, 250 (2023). https://doi.org/10.1007/s00604-023-05823-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05823-x