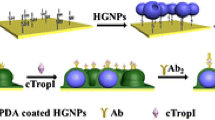
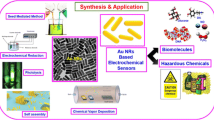
Abstract
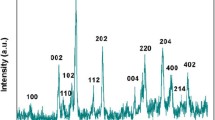
Mulberry-like AuPtAg porous hollow nanorods (PHNR) were facilely synthesized for the first time via a wet chemical method, where Au nanorods (Au NR) behaved as sacrificed template. The anisotropic oriented growth and etching process are involved in this synthesis. Their structural and electronic characteristics were scrutinously examined by TEM, EDS, XPS, and electrochemical techniques. The AuPtAg PHNR provided a large specific surface area and exposed a large number of active sites, showing highly enhanced catalytic activity. On this foundation, a label-free electrochemical immunosensor was developed for myoglobin (Myo) assay based on the AuPtAg PHNR. Further, the built sensor exhibited fast and ultrasensitive responses in a linear range of 0.0001 ~ 1000 ng mL−1 with a low limit of detection (LOD = 0.46 pg mL−1, S/N = 3), and enabled efficient application to human serum samples with acceptable results. Consequently, the developed AuPtAg PHNR–based platform has a broad prospect in practically monitoring Myo and other biomarkers in clinics.
Graphical abstract






Similar content being viewed by others
References
Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore Mensah Y, Elkind MSV, Evenson KR, Eze Nliam C, Ferguson JF, Generoso G, Ho JE, Kalani R, Khan SS, Kissela BM, Knutson KL, Levine DA, Lewis TT, Liu J, Loop MS, Ma J, Mussolino ME, Navaneethan SD, Perak AM, Poudel R, Rezk Hanna M, Roth GA, Schroeder EB, Shah SH, Thacker EL, VanWagner LB, Virani SS, Voecks JH, Wang NY, Yaffe K, Martin SS (2022) Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation 145:e153–e639
White HD, Chew DP (2008) Acute myocardial infarction. Lancet 372:570–584
Alhashemi JA (2006) Diagnostic accuracy of a bedside qualitative immunochromatographic test for acute myocardial infarction. Am J Emerg Med 24:149–155
Li C, Li J, Yang X, Gao L, Jing L, Ma X (2017) A label-free electrochemical aptasensor for sensitive myoglobin detection in meat. Sens Actuators B Chem 242:1239–1245
Shen J, Zhang L, Yuan J, Zhu Y, Cheng H, Zeng Y, Wang J, You X, Yang C, Qu X, Chen H (2021) Digital microfluidic thermal control chip-based multichannel immunosensor for noninvasively detecting acute myocardial infarction. Anal Chem 93:15033–15041
Suprun E, Bulko T, Lisitsa A, Gnedenko O, Ivanov A, Shumyantseva V, Archakov A (2010) Electrochemical nanobiosensor for express diagnosis of acute myocardial infarction in undiluted plasma. Biosens Bioelectron 25:1694–1698
Yoo SS, Kim SY, Kim KS, Hong S, Oh MJ, Nam MG, Kim WJ, Park J, Chung CH, Choe WS, Yoo PJ (2020) Controlling inter-sheet-distance in reduced graphene oxide electrodes for highly sensitive electrochemical impedimetric sensing of myoglobin. Sens Actuators B Chem 305:127477
Piloto AML, Ribeiro DSM, Rodrigues SSM, Santos JLM, Sampaio P, Sales G (2021) Imprinted fluorescent cellulose membranes for the on-site detection of myoglobin in biological media. ACS Appl Bio Mater 4:4224–4235
Wang B, Shi S, Yang X, Wang Y, Qi H, Gao Q, Zhang C (2020) Separation-free electrogenerated chemiluminescence immunoassay incorporating target assistant proximity hybridization and dynamically competitive hybridization of a DNA signal probe. Anal Chem 92:884–891
El Said WA, Fouad DM, El Safty SA (2016) Ultrasensitive label-free detection of cardiac biomarker myoglobin based on surface-enhanced Raman spectroscopy. Sens Actuators B Chem 228:401–409
Adeel M, Rahman MM, Lee JJ (2019) Label-free aptasensor for the detection of cardiac biomarker myoglobin based on gold nanoparticles decorated boron nitride nanosheets. Biosens Bioelectron 126:143–150
Kumar V, Brent JR, Shorie M, Kaur H, Chadha G, Thomas AG, Lewis EA, Rooney AP, Nguyen L, Zhong XL, Burke MG, Haigh SJ, Walton A, McNaughter PD, Tedstone AA, Savjani N, Muryn CA, O’Brien P, Ganguli AK, Lewis DJ, Sabherwal P (2016) Nanostructured aptamer-functionalized black phosphorus sensing platform for label-free detection of myoglobin, a cardiovascular disease biomarker. ACS Appl Mater Interfaces 8:22860–22868
Biswas S, Lan Q, Li C, Xia X (2022) Morphologically flex Sm-MOF based electrochemical immunosensor for ultrasensitive detection of a colon cancer biomarker. Anal Chem 94:3013–3019
Ma E, Wang P, Yang Q, Yu H, Pei F, Li Y, Liu Q, Dong Y (2019) Electrochemical immunosensor based on MoS2 NFs/Au@AgPt YNCs as signal amplification label for sensitive detection of CEA. Biosens Bioelectron 142:111580
Joseph XB, Kogularasu S, Wang SF, Sheu JK (2021) Hydrothermal-dependent synthesis of exfoliated nickel cobaltite layers for simultaneous determination of IARC group 2B, 3B carcinogens. ACS Appl Nano Mater 4:12788–12797
Sriram B, Kogularasu S, Hsu YF, Wang SF, Sheu JK (2022) Fabrication of praseodymium vanadate nanoparticles on disposable strip for rapid and real-time amperometric sensing of arsenic drug roxarsone. Inorg Chem 61:16370–16379
Tang C, Zhang J, Chen D, He J, Wang A, Feng J (2022) Ultrasensitive label-free electrochemical immunosensor of NT-proBNP biomarker based on branched AuPd nanocrystals/N-doped honeycombed porous carbon. Bioelectrochemistry 148:108225
Cui F, Zhou Z, Feng H, Zhou HS (2020) Disposable polyurethane nanospiked gold electrode-based label-free electrochemical immunosensor for clostridium difficile. ACS Appl Nano Mater 3:357–363
Qiu J, Jiang P, Wang C, Chu Y, Zhang Y, Wang Y, Zhang M, Han L (2022) Lys-AuNPs@MoS2 nanocomposite self-assembled microfluidic immunoassay biochip for ultrasensitive detection of multiplex biomarkers for cardiovascular diseases. Anal Chem 94:4720–4728
Sheikhzadeh E, Beni V, Zourob M (2021) Nanomaterial application in bio/sensors for the detection of infectious diseases. Talanta 230:122026
Jiang T, Song Y, Wei T, Li H, Du D, Zhu M, Lin Y (2016) Sensitive detection of Escherichia coli O157:H7 using Pt–Au bimetal nanoparticles with peroxidase-like amplification. Biosens Bioelectron 77:687–694
Karthikeyan B, Murugavelu M (2012) Nano bimetallic Ag/Pt system as efficient opto and electrochemical sensing platform towards adenine. Sens Actuators B Chem 163:216–223
Yang H, Hou J, Wang Z, Zhang T, Xu C (2018) An ultrasensitive biosensor for superoxide anion based on hollow porous PtAg nanospheres. Biosens Bioelectron 117:429–435
Jia Y, Li Y, Zhang S, Wang P, Liu Q, Dong Y (2020) Mulberry-like Au@PtPd porous nanorods composites as signal amplifiers for sensitive detection of CEA. Biosens Bioelectron 149:111842
Chen C, Zhou X, Wang Z, Han J, Chen S (2022) Core–shell Au@PtAg modified TiO2–Ti3C2 heterostructure and target-triggered DNAzyme cascade amplification for photoelectrochemical detection of ochratoxin A. Anal Chim Acta 1216:339943
Zhou X, Zhang W, Wang Z, Han J, Xie G, Chen S (2020) Ultrasensitive aptasensing of insulin based on hollow porous C3N4/S2O82−/AuPtAg ECL ternary system and DNA walker amplification. Biosens Bioelectron 148:111795
Shi Y, Wang A, Yuan P, Zhang L, Luo X, Feng J (2018) Highly sensitive label-free amperometric immunoassay of prostate specific antigen using hollow dendritic AuPtAg alloyed nanocrystals. Biosens Bioelectron 111:47–51
Banan Sadeghian R, Han J, Ostrovidov S, Salehi S, Bahraminejad B, Ahadian S, Chen M, Khademhosseini A (2017) Macroporous mesh of nanoporous gold in electrochemical monitoring of superoxide release from skeletal muscle cells. Biosens Bioelectron 88:41–47
Yang J, Cho M, Lee Y (2016) Synthesis of hierarchical NiCo2O4 hollow nanorods via sacrificial-template accelerate hydrolysis for electrochemical glucose oxidation. Biosens Bioelectron 75:15–22
Guo X, Ye W, Zhu R, Wang W, Xie F, Sun H, Zhao Q, Ding Y, Yang J (2014) Gold nanorod-templated synthesis of polymetallic hollow nanostructures with enhanced electrocatalytic performance. Nanoscale 6:11732–11737
Xue M, Tan Y (2014) Hollow alloy nanostructures templated by Au nanorods: synthesis, mechanistic insights, and electrocatalytic activity. Nanoscale 6:12500–12514
Nikoobakht B, El Sayed M (2003) Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem Mater 15:1957–1962
Chen M, Huang Z, Ye X, Zhang L, Feng J, Wang A (2023) Caffeine derived graphene-wrapped Fe3C nanoparticles entrapped in hierarchically porous FeNC nanosheets for boosting oxygen reduction reaction. J Colloid Interface Sci 637:216–224
Liu L, Wu D, Zhang L, Feng J, Wang A (2023) FeCo alloy entrapped in N-doped graphitic carbon nanotubes-on-nanosheets prepared by coordination-induced pyrolysis for oxygen reduction reaction and rechargeable Zn-air battery. J Colloid Interface Sci 639:424–433
Sreeprasad TS, Samal AK, Pradeep T (2007) Body- or tip-controlled reactivity of gold nanorods and their conversion to particles through other anisotropic structures. Langmuir 23:9463–9471
Feng Y, He J, Jiang L, Chen D, Wang A, Feng J (2022) Novel sandwich-typed electrochemical immunosensing of C-reactive protein using multiply twinned AuPtRh nanobead chains and nitrogen-rich porous carbon nanospheres decorated with Au nanoparticles. Sens Actuators B Chem 358:131518
Cheng D, Zhou Z, Shang S, Wang H, Guan H, Yang H, Liu Y (2022) Electrochemical immunosensor for highly sensitive detection of cTnI via in-situ initiated ROP signal amplification strategy. Anal Chim Acta 1219:340032
Ge X, Feng Y, Cen S, Wang A, Mei L, Luo X, Feng J (2021) A label-free electrochemical immnunosensor based on signal magnification of oxygen reduction reaction catalyzed by uniform PtCo nanodendrites for highly sensitive detection of carbohydrate antigen 15–3. Anal Chim Acta 1176:338750
Yola ML, Atar N (2020) Amperometric galectin-3 immunosensor-based gold nanoparticle-functionalized graphitic carbon nitride nanosheets and core–shell Ti-MOF@COFs composites. Nanoscale 12:19824–19832
Wang B, Mei L, Ma Y, Xu Y, Ren S, Cao J, Liu Y, Zhao W (2018) Photoelectrochemical-chemical-chemical redox cycling for advanced signal amplification: proof-of-concept toward ultrasensitive photoelectrochemical bioanalysis. Anal Chem 90:12347–12351
Singh S, Tuteja SK, Sillu D, Deep A, Suri CR (2016) Gold nanoparticles-reduced graphene oxide based electrochemical immunosensor for the cardiac biomarker myoglobin. Microchim Acta 183:1729–1738
Zhang J, Tang C, Chen D, Jiang L, Wang A, Feng J (2022) Ultrasensitive label-free sandwich immunoassay of cardiac biomarker myoglobin using meso-SiO2@ploydapamine@PtPd nanocrystals and PtNi nanodendrites for effective signal amplification. Appl Surf Sci:155216
Tuteja SK, Chen R, Kukkar M, Song CK, Mutreja R, Singh S, Paul AK, Lee H, Kim K-H, Deep A, Suri CR (2016) A label-free electrochemical immunosensor for the detection of cardiac marker using graphene quantum dots (GQDs). Biosens Bioelectron 86:548–556
Wang Y, Hong Y, Wang M, Zhu Y (2022) Multifunctional nanolabelsbased on polydopamine nanospheres for sensitive alpha fetoprotein electrochemical detection. ACS Appl Nano Mater 5:1588–1599
Tang D, Yang X, Wang B, Ding Y, Xu S, Liu J, Peng Y, Yu X, Su Z, Qin X (2021) One-step electrochemical growth of 2D/3D Zn(II)-MOF hybrid nanocomposites on an electrode and utilization of a PtNPs@2D MOF nanocatalyst for electrochemical immunoassay. ACS Appl Mater Interfaces 13:46225–46232
Acknowledgements
This research was supported by Zhejiang Public Welfare Technology Application Research Project (LGG19B050001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Research Highlights
• AuPtAg PHNR was prepared for the first time by using Au NR as sacrificed template.
• The anisotropic oriented growth and etching process were involved.
• The AuPtAg PHNR provided a large specific surface area, attractive electronic effects and high catalytic activity.
• The immunosensor showed excellent performances for ultrasensitive detection of Myo.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, C., Wang, AJ., Feng, JJ. et al. Mulberry-like porous-hollow AuPtAg nanorods for electrochemical immunosensing of biomarker myoglobin. Microchim Acta 190, 233 (2023). https://doi.org/10.1007/s00604-023-05802-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05802-2