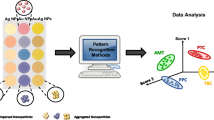
Abstract
A nanozyme sensor array based on the ssDNA-distensible C3N4 nanosheet sensor elements for discriminating multiple mycotoxins commonly existing in contaminated cereals has been explored. The sensor array exploited (a) three DNA nonspecific sequences (A40, T40, C40) absorbed on the C3N4 nanosheets as sensor elements catalyzing the oxidation of TMB; (b) the presence of five mycotoxins affected the catalytic activity of three nanozymes with various degrees. The parameter (A0-A) was employed as the signal output to obtain the response patterns for different mycotoxins with the same concentration where A0 and A were the absorption peak values at 650 nm of oxTMB in the absence and presence of target mycotoxins, respectively. After the raw data was subjected to principal component analysis, 3D canonical score plots were obtained. The sensor array was capable of separating five mycotoxins from each other with 100% accuracy even if the concentration of the mycotoxins was as low as 1 nM. Moreover, the array performed well in discriminating the mycotoxin mixtures with different ratios. Importantly, the practicality of this sensor array was demonstrated by discriminating the five mycotoxins spiking in corn-free samples in 3D canonical score plots, validating that the sensor array can act as a flexible detection tool for food safety.
Graphical abstract
A nanozyme sensor array was developed based on the ssDNA-distensible C3N4 NSs sensor elements for discriminating muitiple mycotoxins.









Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ginter-Hanselmayer G, Nenof P (2019) Clinically relevant mycoses dermatomycoses. Springer. https://doi.org/10.1007/978-3-319-92300-0_10
Hussein H, Brasel JM (2001) Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 167:101–134. https://doi.org/10.1016/S0300-483X(01)00471-1
Ostry V, Malir F, Toman J, Grosse Y (2017) Mycotoxins as human carcinogens-the IARC monographs classification. Mycotoxin Res 33:65–73. https://doi.org/10.1007/s12550-016-0265-7
Yan J, Hu W, You K, Ma Z, Xu Y, Li Y, He Q (2020) Biosynthetic mycotoxin conjugate mimetics-mediated green strategy for multiplex mycotoxin immunochromatographic assay. J Agric Food Chem 68:2193–2200. https://doi.org/10.1021/acs.jafc.9b06383
Christiane GD, Timothy J, Gerd S (2019) Global mycotoxin occurrence in feed: a ten-year survey. Toxins 11:375. https://doi.org/10.3390/toxins11070375
Sun J, Li W, Zhu X, Jiao S, Chang Y, Wang S, Dai S, Xu R, Dou M, Li Q, Li J (2021) A novel multiplex mycotoxin surface-enhanced raman spectroscopy immunoassay using functional gold nanotags on a silica photonic crystal microsphere biochip. J Agric Food Chem 69:11494–11501. https://doi.org/10.1021/acs.jafc.1c03469
Dong S, Yan J, Zhou S, Zhou Q (2022) Mycotoxins detection based on electrochemical approaches. Electroanalysis 34:132–147. https://doi.org/10.1002/elan.202100349
Wu Z, Xu E, Chughtai MFJ, Jin Z, Irudayaraj J (2017) Highly sensitive fluorescence sensing of zearalenone using a novel aptasensor based on upconverting nanoparticles. Food Chem 230:673–680. https://doi.org/10.1016/j.foodchem.2017.03.100
Delaney EAP, Prashant SD, Richard AM (2020) Intrinsic “turn-on” aptasensor detection of ochratoxin a using energy-transfer fluorescence. J Agric Food Chem 68:2249–2255. https://doi.org/10.1021/acs.jafc.9b07391
Jiang D, Huang C, Shao L, Wang X, Jiao Y, Li W, Chen J, Xu X (2020) Magneto-controlled aptasensor for simultaneous detection of ochratoxin A and fumonisin B1 using inductively coupled plasma mass spectrometry with multiple metal nanoparticles as element labels. Anal Chim Acta 1127:182–189. https://doi.org/10.1016/j.aca.2020.06.057
Yang Y, Li W, Shen P, Liu R, Li Y, Xu J, Zheng Q, Zhang Y, Li J, Zheng T (2017) Aptamer fluorescence signal recovery screening for multiplex mycotoxins in cereal samples based on photonic crystal microsphere suspension array. Sensors and Actuators B 248:351–358. https://doi.org/10.1016/j.snb.2017.04.004
Goud KY, Reddy KK, Satyanarayana M, Vengatajalabathy G (2020) A review on recent developments in optical and electrochemical aptamer-based assays for mycotoxins using advanced nanomaterials. Microchim Acta 187:1–32. https://doi.org/10.1007/s00604-019-4034-0
He D, Wu Z, Cui B, Jin Z, Xu E (2020) A fluorometric method for aptamer-based simultaneous determination of two kinds of the fusarium mycotoxins zearalenone and fumonisin B1 making use of gold nanorods and upconversion nanoparticles. Microchim Acta 187:1–8. https://doi.org/10.1007/s00604-020-04236-4
Wu S, Duan N, Ma X, Xia Y, Wang H, Wang Z, Zhang Q (2012) Multiplexed fluorescence resonance energy transfer aptasensor between upconversion nanoparticles and graphene oxide for the simultaneous determination of mycotoxins. Anal Chem 84:6263–6270. https://doi.org/10.1021/ac301534w
Qin L, Wang X, Liu Y, Wei H (2018) 2D-MOF nanozyme sensor arrays for probing phosphates and their enzymatic hydrolysis. Anal Chem 90:9983–9989. https://doi.org/10.1021/acs.analchem.8b02428
Li Y, Wang S, Liu Q, Chen Z (2019) A chrono-colorimetric sensor array for differentiation of catechins based on silver nitrate-induced metallization of gold nanoparticles at different reaction time intervals. ACS Sustainable Chem Eng 7:17306–17312. https://doi.org/10.1021/acssuschemeng.9b04154
Liu C, You X, Lu D, Shi G, Deng J, Zhou T (2020) Gelsolin encountering Ag nanorods/triangles: an aggregation based colorimetric sensor array for in vivo monitoring the cerebrospinal Aβ42% as an indicator of Cd2+ exposure-related Alzheimer’s disease pathogenesis. ACS Appl Bio Mater 3:7965–7973. https://doi.org/10.1021/acsabm.0c01078
Mao J, Lu Y, Chang N, Yang J, Zhang S, Liu Y (2016) Multidimensional colorimetric sensor array for discrimination of proteins. Biosens Bioelectron 86:56–61. https://doi.org/10.1016/j.bios.2016.06.040
Sun W, Lu Y, Mao J, Chang N, Yang J, Liu Y (2015) Multidimensional sensor for pattern recognition of proteins based on DNA-Gold nanoparticles conjugates. Anal Chem 87:3354–3359. https://doi.org/10.1021/ac504587h
Wu S, Guo D, Xu X, Pan J, Niu X (2020) Colorimetric quantification and discrimination of phenolic pollutants based on peroxidase-like Fe3O4 nanoparticles. Sens Actuators, B Chem 303:127225. https://doi.org/10.1016/j.snb.2019.127225
Li S, Liu X, Liu Q, Chen Z (2020) Colorimetric differentiation of flavonoids based on effective reactivation of acetylcholinesterase induced by different affinities between flavonoids and metal ions. Anal Chem 92:3361–3365. https://doi.org/10.1021/acs.analchem.9b05378
Wang Z, Xu C, Lu Y, Chen X, Yuan H, Wei G, Ye G, Chen J (2017) Fluorescence sensor array based on amino acid derived carbon dots for pattern-based detection of toxic metal ions. Sensors Actuators B Chem 241:1324–1330. https://doi.org/10.1016/j.snb.2016.09.186
Xu W, Ren C, Teoh CL, Peng J, Gadre SH, Rhee HW, Lee CLK, Chang YT (2014) An artificial tongue fluorescent sensor array for identification and quantitation of various heavy metal ions. Anal Chem 86:8763–8769. https://doi.org/10.1021/ac501953z
Li M, Guan Y, Ding C, Chen Z, Ren J, Qu X (2016) An ultrathin graphitic carbon nitride nanosheet: a novel inhibitor of metal-induced amyloid aggregation associated with Alzheimer’s disease. J Mater Chem B 4:4072–4075. https://doi.org/10.1039/C6TB01215A
Lv Y, Chen S, Shen Y, Ji J, Zhou Q, Liu S, Zhang Y (2018) Competitive multiple-mechanism-driven electrochemiluminescent detection of 8-hydroxy-2′-deoxyguanosine. J Am Chem Soc 140:2801–2804. https://doi.org/10.1021/jacs.8b00515
Wang X, Qin L, Lin M, Xing H, Wei H (2019) Fluorescent graphitic carbon nitride-based nanozymes with peroxidase-like activities for ratiometric biosensing. Anal Chem 91:10648–10656. https://doi.org/10.1021/acs.analchem.9b01884
Xiong Y, Li W, Wen Q, Xu D, Ren J, Lin Q (2022) Aptamer-engineered nanomaterials to aid in mycotoxin determination. Food Control 135:108661. https://doi.org/10.1016/j.foodcont.2021.108661
Wang Q, Wang W, Lei J, Xu N, Gao F, Ju H (2013) Fluorescence quenching of carbon nitride nanosheet through its interaction with DNA for versatile fluorescence sensing. Anal Chem 85:12182–12188. https://doi.org/10.1021/ac403646n
Wang YM, Liu JW, Adkins GB, Shen W, Trinh MP, Duan LY, Jiang JH, Zhong W (2017) Enhancement of the intrinsic peroxidase-like activity of graphitic carbon nitride nanosheets by ssDNAs and its application for detection of exosomes. Anal Chem 89:12327–12333. https://doi.org/10.1021/acs.analchem.7b03335
Liu MX, Zhang H, Zhang XW, Chen S, Yu YL, Wang JH (2021) Nanozyme sensor array plus solvent-mediated signal amplification strategy for ultrasensitive ratiometric fluorescence detection of exosomal proteins and cancer identification. Anal Chem 93:9002–9010. https://doi.org/10.1021/acs.analchem.1c02010
Avantaggiato G, Greco D, Damascelli A, Solfrizzo M, Visconti A (2014) Assessment of multi-mycotoxin adsorption efficacy of grape pomace. J Agric Food Chem 62:497–507. https://doi.org/10.1021/jf404179h
Wang J, Yu L, Wang Z, Wei W, Wang K, Wei X (2021) Constructing 0D/2D Z-scheme heterojunction of CdS/g-C3N4 with enhanced photocatalytic activity for H2 evolution. Catal Lett 151:3550–3561. https://doi.org/10.1007/s10562-021-03579-8
Ma TY, Tang Y, Dai S, Qiao S (2014) Proton-functionalized two-dimensional graphitic carbon nitride nanosheet: an excellent metal-/label-free biosensing platform. Small 10:2382–2389. https://doi.org/10.1002/smll.201303827
Guo H, Ji J, Wang J, Sun X (2020) Co-contamination and interaction of fungal toxins and other environmental toxins. Trends Food Sci Technol 103:162–178. https://doi.org/10.1016/j.tifs.2020.06.021
Zong C, Jiang F, Wang X, Li P, Xu L, Yang H (2021) Imaging sensor array coupled with dual-signal amplification strategy for ultrasensitive chemiluminescence immunoassay of multiple mycotoxins. Biosens Bioelectron 177:112998. https://doi.org/10.1016/j.bios.2021.112998
Zong C, Jiang F, Wang X, Li P, Xu L, Yang H (2021) Imaging sensor array coupled with dual-signal amplification strategy for ultrasensitive chemiluminescence immunoassay of multiple mycotoxins. Biosens Bioelectron 177:112998. https://doi.org/10.1016/j.bios.2021.112998
Nolan P, Auer S, Spehar A, Oplatowska-Stachowiak M, Campbell K (2021) Evaluation of mass sensitive micro-array biosensors for their feasibility in multiplex detection of low molecular weight toxins using mycotoxins as model compounds. Talanta 222:121521. https://doi.org/10.1016/j.talanta.2020.121521
Wang YK, Yan YX, Ji WH, Wang H, Li SQ, Zou Q, Sun JH (2013) Rapid simultaneous quantification of zearalenone and fumonisin B1 in corn and wheat by lateral flow dual immunoassay. J Agric Food Chem 61:5031–5036. https://doi.org/10.1021/jf400803q
Funding
This project was supported by the National Natural Science Foundation of China (grant Nos. 21605100) and the Natural Science Foundation of Shandong Province (ZR2021MB066, ZR2020KB020).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Jing Zhu has received research grants from the National Natural Science Foundation of China and the Natural Science Foundation of Shandong Province (21605100 and ZR2021MB066). Rongmei Kong has received research grants from the Natural Science Foundation of Shandong Province (ZR2020KB020). The authors declare that they have no conflict of interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, J., Xu, W., Yang, Y. et al. ssDNA-C3N4 conjugates-based nanozyme sensor array for discriminating mycotoxins. Microchim Acta 190, 6 (2023). https://doi.org/10.1007/s00604-022-05593-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05593-y