Abstract
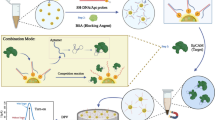
A new type of aptamer-functionalized pH-responsive polymer-modified magnetic nanoparticles (ApMNPs) is introduced for specific enrichment and sensitive determination of lactoferrin (Lf) in complex matrixes. In the construction, Fe3O4@3-(Triethoxysilyl)propylmethacrylate@poly(4-Vinyl-1, 3-dioxolan-2-one-acrylic acid) (Fe3O4@MPS@p(VEC-AA)) were synthesized as pH-responsive polymer-modified magnetic nanoparticles (pMNPs) through free radical polymerization to increase the tunable interaction. Lf-binding aptamers were conjugated onto pMNPs through the reaction of amino-group in aptamer and epoxide-group in VEC, innovatively applied to prepare Lf-ApMNPs. On the basis of the synergistic effect of specific affinity of aptamer on Lf and tunable hydrophobic/hydrophilic property of pH-responsive polymer, Lf-ApMNPs presented good selectivity toward Lf, excellent adsorption capacity (as high as 233.9 mg g−1), as well as good recoveries in the range 93.6–99.6% in Lf-related nutrition samples. Significantly, the introduction of pH-responsive monomer (AA) effectively regulated the adsorption–desorption process of Lf, with the function similar to a switch. Moreover, the good performances of Ct-ApMNPs toward α-Chymotrypsin showed that ApMNPs exhibited universality to other proteins through easily changing the binding aptamer, thereby offering a facile and efficient approach for specific enrichment and sensitive determination of targets in real biological samples.
Graphical Abstract






Similar content being viewed by others
References
Lönnerdal B, Iyer S (1995) Lactoferrin: molecular structure and biological function. Annu Rev Nutr 15:93–110. https://doi.org/10.1146/annurev.nu.15.070195.000521
Adlerova L, Bartoskova A, Faldyna M (2008) Lactoferrin: a review. Vet Med 53(9):457–468. https://doi.org/10.17221/1978-VETMED
Hao LY, Shan Q, Wei JY, Ma FT, Sun P (2019) Lactoferrin: major physiological functions and applications. Curr Protein Pept Sci 20(2):139–144. https://doi.org/10.2174/1389203719666180514150921
Li JB, Zhu WZ, Luo MR, Ren HH, Tang L, Liao HH, Wang Y (2015) Molecular cloning, expression and purification of lactoferrin from Tibetan sheep mammary gland using a yeast expression system. Protein Expression Purif 109:35–39. https://doi.org/10.1016/j.pep.2015.01.008
Du QY, Lin DQ, Zhang QL, Yao SJ (2014) An integrated expanded bed adsorption process for lactoferrin and immunoglobulin G purification from crude sweet whey. J Chromatogr B 947:201–207. https://doi.org/10.1016/j.jchromb.2013.12.020
Bläckberg L, Hernell O (1980) Isolation of lactoferrin from human whey by a single chromatographic step. FEBS Lett 109(12):180–184. https://doi.org/10.1016/0014-5793(80)81081-7
Teepakorn C, Fiaty K, Charcosset C (2015) Optimization of lactoferrin and bovine serum albumin separation using ion-exchange membrane chromatography. Sep Purif Technol 151:292–302. https://doi.org/10.1016/j.seppur.2015.07.046
Chen L, Guo C, Guan YP, Liu HZ (2007) Isolation of lactoferrin from acid whey by magnetic affinity separation. Sep Purif Technol 56(2):168–174. https://doi.org/10.1016/j.seppur.2007.01.019
Gupta AK, Gupta M (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26(18):3995–4021. https://doi.org/10.1016/j.biomaterials.2004.10.012
Espinoza SS, Cros B, Ávila S, Lezcano G, Dabas P, Vizioli N, Carballo R (2021) Preparation of a biomimetic Cu (II) protoporphyrin magnetic nanocomposite and its application for the selective adsorption of angiotensin I. Microchem J 170:106691. https://doi.org/10.1016/j.microc.2021.106691
Santos MG, de Carvalho DT, Caminitti LB, de Lima BBA, Cavalcanti MHD, dos Santos DFR, Virtuoso LS, Hirata DB, Figueiredo EC (2021) Use of magnetic Fe3O4 nanoparticles coated with bovine serum albumin for the separation of lysozyme from chicken egg white. Food Chem 353:129442. https://doi.org/10.1016/j.foodchem.2021.129442
Zhao LP, Li LS, Zhu C, Ghulam M, Qu F (2020) pH-responsive polymer assisted aptamer functionalized magnetic nanoparticles for specific adsorption and content determination of proteins. Anal Chim Acta 1097:161–168. https://doi.org/10.1016/j.aca.2019.11.001
Yang L, Liu H (2013) Stimuli-responsive magnetic particles and their applications in biomedical field. Powder Technol 240:54–65. https://doi.org/10.1016/j.powtec.2012.07.007
Gao F, Qi Q, Wu X, Wu XL, Yu JX, Yao J, Cao ZH, Mi YF, Cui QM (2021) Multifunctional poly (quaternary ammonium)/Fe3O4 composite nanogels for integration of antibacterial and degradable magnetic redox-responsive properties. Colloids Surf A 615:126235. https://doi.org/10.1016/j.colsurfa.2021.126235
Liu HB, Yang XH, Wang JX, Meng QJ, Qian LW, Wu HW, Duan C, Li ZJ, Zhou HW (2020) Gas responsive cellulose fibers for capturing and releasing of dyes and proteins from water by packing a smart separation column. Cellulose 27(12):7127–7138. https://doi.org/10.1007/s10570-020-03277-5
Guo J, Wang NJ, Peng L, Wu JJ, Ye QQ, Feng AC, Wang ZP, Zhang C, Xing XH, Yuan JY (2016) Electrochemically-responsive magnetic nanoparticles for reversible protein adsorption. J Mater Chem B 4(22):4009–4016. https://doi.org/10.1039/c6tb00259e
Fan JP, Yu JX, Yang XM, Zhang XH, Yuan TT, Peng HL (2018) Preparation, characterization, and application of multiple stimuli-responsive rattle-type magnetic hollow molecular imprinted poly (ionic liquids) nanospheres (Fe3O4@void@PILMIP) for specific recognition of protein. Chem Eng J 337:722–732. https://doi.org/10.1016/j.cej.2017.12.159
Jiang DD, Hu TT, Zheng HJ, Xu GX, Jia Q (2018) Aptamer-functionalized magnetic conjugated organic framework for selective extraction of traces of hydroxylated polychlorinated biphenyls in human serum. Chem-Eur J 24(41):10390–10396. https://doi.org/10.1002/chem.201800092
Charbgoo F, Soltani F, Taghdisi SM, Abnous K, Ramezani M (2016) Nanoparticles application in high sensitive aptasensor design. TrAC Trends Anal Chem 85:85–97. https://doi.org/10.1016/j.trac.2016.08.008
Abrego-Martinez JC, Jafari M, Chergui S, Pavel C, Che DP, Siaj M (2022) Aptamer-based electrochemical biosensor for rapid detection of SARS-CoV-2: nanoscale electrode-aptamer-SARS-CoV-2 imaging by photo-induced force microscopy. Biosens Bioelectron 195:113595. https://doi.org/10.1016/j.bios.2021.113595
Wang ZD, Huang CW, Sun NR, Deng CH (2021) Advances in aptamer-based nanomaterials for separation and analysis of non-genetic biomarkers in biofluids. Sci Chi Chem 64(6):932–947. https://doi.org/10.1007/s11426-020-9955-y
Shen MM, Wang YY, Kan XW (2021) Dual-recognition colorimetric sensing of thrombin based on surface-imprinted aptamer-Fe3O4. J Mater Chem B 9(20):4249–4256. https://doi.org/10.1039/d1tb00565k
Zhang XY, Zhu SC, Deng CH, Zhang XM (2012) Highly sensitive thrombin detection by matrix assisted laser desorption ionization-time of flight mass spectrometry with aptamer functionalized core-shell Fe3O4@C@Au magnetic microspheres. Talanta 88:295–302. https://doi.org/10.1016/j.talanta.2011.10.044
Luo RP, Zhou XR, Chen Y, Tuo SC, Jiang FL, Niu XD, Pan FG, Wang HS (2019) Lysozyme aptamer-functionalized magnetic nanoparticles for the purification of lysozyme from chicken egg white. Foods 8(2):67. https://doi.org/10.3390/foods8020067
Su Y, Xue TT, Wu LP, Hu YL, Wang J, Xu QY, Chen YY, Lin ZK (2019) Label-free detection of biomarker alpha fetoprotein in serum by ssDNA aptamer functionalized magnetic nanoparticles. Nanotechnology 31(9):095104. https://doi.org/10.1088/1361-6528/ab57f7
Zhu C, Li LS, Yang G, Irfan M, Wang ZJ, Fang SB, Qu F (2019) High-efficiency selection of aptamers for bovine lactoferrin by capillary electrophoresis and its aptasensor application in milk powder. Talanta 205:120088. https://doi.org/10.1016/j.talanta.2019.06.088
Xiao P, Lv XF, Wang SS, Iqbal J, Qing H, Li Q, Deng YL (2013) An aptamer-based trypsin reactor for on-line protein digestion with electrospray ionization tandem mass spectrometry. Anal Biochem 441(2):123–132. https://doi.org/10.1016/j.ab.2013.06.012
Zhao LP, Yang G, Li LS, Zhu C, Ma Y, Qu F (2020) Aptamer-functionalized magnetic nanoparticles conjugated organic framework for immobilization of acetylcholinesterase and its application in inhibitors screening. Anal Chim Acta 1140:228–235. https://doi.org/10.1016/j.aca.2020.10.024
Gao RX, Zhang LL, Hao Y, Cui XH, Liu DC, Zhang M, Tang YH (2015) Novel polydopamine imprinting layers coated magnetic carbon nanotubes for specific separation of lysozyme from egg white. Talanta 144:1125–1132. https://doi.org/10.1016/j.talanta.2015.07.090
Huang Q, Li Y, Zhang H, Song XW, Li QW, Cao XL, Li ZQ (2009) Environmental-responsive behavior of poly(acrylic acid). Acta Chim Sinic 67:2421–2426. https://doi.org/10.3321/j.issn:0567-7351.2009.21.005
Lai BH, Chang CH, Yeh CC, Chen DH (2013) Direct binding of concanvalin A onto iron oxide nanoparticles for fast magnetic selective separation of lactoferrin. Sep Purif Technol 108:83–88. https://doi.org/10.1016/j.seppur.2013.02.020
Zhang JL, Di W, Gong PM, Lin K, Lyu LZ, Zhang LW, Han X, Ma Y (2018) Direct and fast capture lactoferrin from cheese whey on nanoparticles of Fe3O4 combined with concanavalin A. Food Chem 274:314–318. https://doi.org/10.1016/j.foodchem.2018.08.115
Baieli MF, Urtasun N, Miranda MV, Franzreb M (2014) Isolation of lactoferrin from whey by dye-affinity chromatography with Yellow HE-4R attached to chitosan mini-spheres. Int Dairy J 39(1):53–59. https://doi.org/10.1016/j.idairyj.2014.03.014
Meyer A, Berensmeier S, Franzreb M (2007) Direct capture of lactoferrin from whey using magnetic micro-ion exchangers in combination with high-gradient magnetic separation. React Funct Polym 67(12):1577–1588. https://doi.org/10.1016/j.reactfunctpolym.2007.07.038
Huang YH, Wang YZ, Pan Q, Wang Y, Ding XQ, Xu KJ, Li N, Wen Q (2015) Magnetic graphene oxide modified with choline chloride-based deep eutectic solvent for the solid-phase extraction of protein. Anal Chim Acta 877:90–99. https://doi.org/10.1016/j.aca.2015.03.048
Acknowledgements
All authors made the contributions to the manuscript and had approved the final version of the script. We thank the support of the National Natural Science Foundation of China [no. 21874010, 21827810], Natural Science Foundation of Beijing Municipality [no. 7222310], Natural Science Foundation of Shandong Province (no. ZR2022QB207), Supported by the Taishan Scholars Program, the Agricultural scientific and technological innovation project of the Shandong Academy of Agricultural Sciences (CXGC2021B14, CXGC2022E05), and the Young Elite Scientist Sponsorship Program of the Beijing Association for Science and Technology.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Y., Zhao, L., Li, L. et al. Aptamer-functionalized pH-responsive polymer-modified magnetic nanoparticles for specific enrichment and sensitive determination of lactoferrin. Microchim Acta 190, 26 (2023). https://doi.org/10.1007/s00604-022-05589-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05589-8