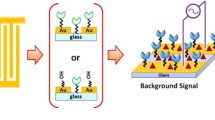
Abstract
Cysticercosis, caused by Taenia solium infection, is a leading cause of acquired epilepsy in many developing countries. Several types of immunoassays have been developed for the detection of Taenia solium infection in both infected humans and livestock animals. However, these methods require central laboratory facilities and are both time- and labor-consuming with longer than desired turnaround time. In this work, we demonstrated that AC electrokinetics (ACEK) capacitive sensing can be used to realize point-of-care immunosensor in general, with the on-site screening of Taenia solium infection as an example here. The sensor employs interdigitated microelectrodes (IDME) functionalized with a recombinant Taenia solium antigen, rT24H, to detect anti-rT24H antibodies in clinical serum samples. ACEK capacitive sensing method interrogates the IDME sensors with a special AC signal, which serves the dual purposes of enriching target antibodies by ACEK effects and directly measuring the capacitance change induced by specific binding. First, to characterize the ACEK biosensor as an immunosensor in general, IgG in phosphate-buffered saline buffer was tested against IDME sensors functionalized with anti-IgG. The limit of detection of the sensor was 24.1 fg/mL, and the linear dynamic range was 0.1–100 pg/mL. To test the clinical usage of this sensor, ACEK capacitive sensors with rT24H probe were used to test clinical serum samples from patients with or without Taenia solium infection. The diagnostic sensitivity of the ACEK capacitive sensor for Taenia solium infection was found to be 88.24%. ACEK capacitive immunosensors have shown good potential for point-of-care diagnostics.
Graphical Abstract






Similar content being viewed by others
Abbreviations
- ACEK:
-
AC electrokinetics
- ACET:
-
AC electrothermal effect
- ELISA:
-
Enzyme-linked immunosorbent assay
- EITB:
-
Enzyme-linked immunoelectrotransfer blot
- IDME:
-
Interdigitated microelectrodes
- IgG:
-
Immunoglobulin G
- LLGP:
-
Lentil lectin-bound glycoproteins
- POC:
-
Point-of-care
- EDL:
-
Electrical double layer
- EIS:
-
Electrochemical impedance spectroscopy
References
Garcia HH, Gilman RH, Catacora M et al (1997) Serologic evolution of neurocysticercosis patients after antiparasitic therapy. J Infect Dis 175:486–489. https://doi.org/10.1093/infdis/175.2.486
Garcia HH, del Brutto OH (2000) Taenia solium cysticercosis. Infect Dis Clin North Am 14:97–119. https://doi.org/10.1016/S0891-5520(05)70220-8
Coyle CM, Mahanty S, Zunt JR et al (2012) Neurocysticercosis: neglected but not forgotten. PLoS Negl Trop Dis 6:9–11. https://doi.org/10.1371/journal.pntd.0001500
del Brutto OH (2012) Neurocysticercosis: a review. Scientific World Journal 2012:159821. https://doi.org/10.1100/2012/159821
Rosas N, Sotelo J, Nieto D (1986) ELISA in the diagnosis of neurocysticercosis. Arch Neurol 43:353–356. https://doi.org/10.1001/archneur.1986.00520040039016
Tsang VCW, Brand JA, Boyer AE (1989) An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis 159:50–59. https://doi.org/10.1093/infdis/159.1.50
Feldman M, Plancarte A, Sandoval M et al (1990) Comparison of two assays (EIA and EITB) and two samples (saliva and serum) for the diagnosis of neurocysticercosis. Trans R Soc Trop Med Hyg 84:559–562. https://doi.org/10.1016/0035-9203(90)90040-L
Corstjens PLAM, de Dood CJ, Priest JW et al (2014) Feasibility of a lateral flow test for neurocysticercosis using novel up-converting nanomaterials and a lightweight strip analyzer. PLoS Negl Trop Dis 8:e2944
Hancock K, Pattabhi S, Whitfield FW et al (2006) Characterization and cloning of T24, a Taenia solium antigen diagnostic for cysticercosis. Mol Biochem Parasitol 147:109–117. https://doi.org/10.1016/j.molbiopara.2006.02.004
Handali S, Klarman M, Gaspard AN et al (2010) Multiantigen print immunoassay for comparison of diagnostic antigens for Taenia solium cysticercosis and taeniasis. Clin Vaccine Immunol 17:68–72. https://doi.org/10.1128/CVI.00339-09
Lee YM, Handali S, Hancock K et al (2011) Serologic diagnosis of human Taenia solium cysticercosis by using recombinant and synthetic antigens in QuickELISA™. Am J Trop Med Hyg 84:587–593. https://doi.org/10.4269/ajtmh.2011.10-0079
Handali S, Pattabhi S, Lee YM et al (2010) Development and evaluation of porcine cysticercosis quickelisa ™ in triturus® EIA analyzer. J Immunoassay Immunochem 31:60–70. https://doi.org/10.1080/15321810903405068
Bustos JA, Ninaquispe BE, Rodriguez S et al (2019) Performance of a sandwich antigen-detection ELISA for the diagnosis of porcine Taenia solium cysticercosis. Am J Trop Med Hyg 100:604–608. https://doi.org/10.4269/ajtmh.18-0697
Dermauw V, Carabin H, Cissé A et al (2018) Evaluating the recombinant T24H enzyme-linked immunoelectrotransfer blot assay for the diagnosis of neurocysticercosis in a panel of samples from a large community-based randomized control trial in 60 villages in Burkina Faso. Am J Trop Med Hyg 98:565–569. https://doi.org/10.4269/ajtmh.17-0541
Filik H, Avan AA, Altaş Puntar N et al (2022) Electrochemical immunosensor for individual and simultaneous determination of cytokeratin fragment antigen 21-1 and neuron-specific enolase using carbon dots-decorated multiwalled carbon nanotube electrode. Microchem J 183:107990. https://doi.org/10.1016/j.microc.2022.107990
Kim KR, Lee KW, Chun HJ et al (2022) Wash-free operation of smartphone-integrated optical immunosensor using retroreflective microparticles. Biosens Bioelectron 196:113722. https://doi.org/10.1016/j.bios.2021.113722
He Q, Fang Y, Yang H et al (2021) Enhanced performance of a surface plasmon resonance-based immunosensor for the detection of glycocholic acid. Anal Methods 13:1919–1924. https://doi.org/10.1039/d1ay00357g
Peng X, Wang Y, Wen W et al (2021) Simple MoS2-nanofiber paper-based fluorescence immunosensor for point-of-care detection of programmed cell death protein 1. Anal Chem 93:8791–8798. https://doi.org/10.1021/acs.analchem.1c00269
Pal A, Biswas S, Kare SPO et al (2021) Development of an impedimetric immunosensor for machine learning-based detection of endometriosis: a proof of concept. Sens Actuators B Chem 346:130460. https://doi.org/10.1016/j.snb.2021.130460
Sharma P, Chauhan R, Pande V et al (2022) Rapid sensing of Tilletia indica–Teliospore in wheat extract by a piezoelectric label free immunosensor. Bioelectrochemistry 147:108175. https://doi.org/10.1016/j.bioelechem.2022.108175
Oueslati R, Cheng C, Wu J, Chen J (2018) Highly sensitive and specific on-site detection of serum cocaine by a low cost aptasensor. Biosens Bioelectron 108:103–108. https://doi.org/10.1016/j.bios.2018.02.055
Cheng C, Cui H, Wu J, Eda S (2017) A PCR-free point-of-care capacitive immunoassay for influenza A virus. Microchim Acta 184:1649–1657. https://doi.org/10.1007/s00604-017-2140-4
Jiang Y, Huang J, Wu J, Eda S (2022) A rapid, sensitive, and simple-to-use biosensor for on-site detection of attomolar level microrna biomarkers from serum extracellular vesicles. Sens Actuators B Chem 369:132314. https://doi.org/10.1016/j.snb.2022.132314
Lian M, Islam N, Wu J (2007) AC electrothermal manipulation of conductive fluids and particles for lab-chip applications. IET Nanobiotechnol 1:36–43. https://doi.org/10.1049/iet-nbt:20060022
Lian M, Wu J (2009) Microfluidic flow reversal at low frequency by AC electrothermal effect. Microfluid Nanofluidics 7:757–765. https://doi.org/10.1007/s10404-009-0433-6
Ramos A, Morgan H, Green NG, Castellanos A (1999) The role of electrohydrodynamic forces in the dielectrophoretic manipulation and separation of particles. J Electrostat 47:71–81. https://doi.org/10.1016/S0304-3886(99)00031-5
Wu J, Ben Y, Battigelli D, Chang HC (2005) Long-range AC electroosmotic trapping and detection of bioparticles. Ind Eng Chem Res 44:2815–2822. https://doi.org/10.1021/ie049417u
Yang K, Wu J (2010) Numerical study of in situ preconcentration for rapid and sensitive nanoparticle detection. Biomicrofluidics 4:1–15. https://doi.org/10.1063/1.3467446
Salari A, Thompson M (2018) Recent advances in AC electrokinetic sample enrichment techniques for biosensor development. Sens Actuators B Chem 255:3601–3615. https://doi.org/10.1016/j.snb.2017.09.069
Cui H, Cheng C, Lin X et al (2016) Rapid and sensitive detection of small biomolecule by capacitive sensing and low field AC electrothermal effect. Sens Actuators B Chem 226:245–253. https://doi.org/10.1016/j.snb.2015.11.129
Cui H, Li S, Yuan Q et al (2013) An AC electrokinetic impedance immunosensor for rapid detection of tuberculosis. Analyst 138:7188–7196. https://doi.org/10.1039/c3an01112g
Li S, Ren Y, Cui H et al (2015) Alternating current electrokinetics enhanced in situ capacitive immunoassay. Electrophoresis 36:471–474. https://doi.org/10.1002/elps.201400284
Wu J (2008) Interactions of electrical fields with fluids: laboratory-on-a-chip applications. IET Nanobiotechnol 2:14–27. https://doi.org/10.1049/iet-nbt:20070023
Mirzajani H, Cheng C, Wu J et al (2016) Design and characterization of a passive, disposable wireless AC-electroosmotic lab-on-a-film for particle and fluid manipulation. Sens Actuators B Chem 235:330–342. https://doi.org/10.1016/j.snb.2016.05.073
Lin X, Cheng C, Terry P et al (2017) Rapid and sensitive detection of bisphenol a from serum matrix. Biosens Bioelectron 91:104–109. https://doi.org/10.1016/j.bios.2016.12.024
Daniels JS, Pourmand N (2007) Label-free impedance biosensors: opportunities and challenges. Electroanalysis 19:1239–1257. https://doi.org/10.1002/elan.200603855
Sang S, Wang Y, Feng Q et al (2016) Progress of new label-free techniques for biosensors: a review. Crit Rev Biotechnol 36:465–481. https://doi.org/10.3109/07388551.2014.991270
Li S, Yuan Q, Morshed BI et al (2013) Dielectrophoretic responses of DNA and fluorophore in physiological solution by impedimetric characterization. Biosens Bioelectron 41:649–655. https://doi.org/10.1016/j.bios.2012.09.036
Green NG, Ramos A, Morgan H (2000) Ac electrokinetics: a survey of sub-micrometre particle dynamics. J Phys D Appl Phys 33:632–641. https://doi.org/10.1088/0022-3727/33/6/308
Çetin B, Li D (2011) Dielectrophoresis in microfluidics technology. Electrophoresis 32:2410–2427. https://doi.org/10.1002/elps.201100167
Loire S, Kauffmann P, Mezić I, Meinhart CD (2012) A theoretical and experimental study of ac electrothermal flows. J Phys D Appl Phys 45:185301. https://doi.org/10.1088/0022-3727/45/18/185301
Yang K, Islam N, Eda S, Wu J (2017) Optimization of an AC electrokinetics immunoassay lab-chip for biomedical diagnostics. Microfluid Nanofluidics 21:1–11. https://doi.org/10.1007/s10404-017-1867-x
Mirzajani H, Cheng C, Vafaie RH et al (2022) Optimization of ACEK-enhanced, PCB-based biosensor for highly sensitive and rapid detection of bisphenol a in low resource settings. Biosens Bioelectron 196:113745. https://doi.org/10.1016/j.bios.2021.113745
Cheng C, Wu J, Chen J (2019) A highly sensitive aptasensor for on-site detection of lipopolysaccharides in food. Electrophoresis 40:890–896. https://doi.org/10.1002/elps.201800289
Li S, Cui H, Yuan Q et al (2014) AC electrokinetics-enhanced capacitive immunosensor for point-of-care serodiagnosis of infectious diseases. Biosens Bioelectron 51:437–443. https://doi.org/10.1016/j.bios.2013.08.016
Cui H, Cheng C, Wu J, Eda S (2013) Rapid detection of progesterone by commercially available microelectrode chips. 2013 IEEE Sensors 1–4. https://doi.org/10.1109/ICSENS.2013.6688157
Acknowledgements
We thank Prof. Sukwan Handali of the US CDC for providing the antigen rT24H, pooled serum samples, and clinical serum samples.
Funding
This work was supported by The University of Tennessee Interdisciplinary Seed grant, the US NSF CPS/USDA NIFA (Grant No. 2017–67007-26150), and the US DHS (Grant No. D15PC00284). X. Lin received support from China’s National Foreign Expert project (Grant No. G2022165024L) and Natural Science Foundation of Chongqing, China (Grant No. CSTB2022NSCQ-MSX0560).
Author information
Authors and Affiliations
Contributions
J. W. was responsible for the overall investigation and the manuscript reviewing. X. L. and Y. J. were responsible for experiment design, data collecting, and original manuscript writing. S. E. was responsible for sample preparation. W. N. was responsible for original manuscript reviewing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, X., Jiang, Y., Wu, J.J. et al. An alternating current electrokinetics biosensor for rapid on-site serological screening of Taenia solium cysticercosis infection. Microchim Acta 189, 476 (2022). https://doi.org/10.1007/s00604-022-05575-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05575-0