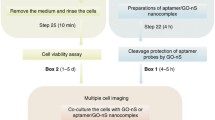
Abstract
Cysteinyl aspartate-specific protease 8 (caspase-8) plays a key role in various biological processes by regulating apoptosis. Therefore, this makes accurate detection and intracellular imaging of caspase-8 of great importance for drug screening, disease diagnosis, and prognostication. Here, by designing a reduced graphene oxide (rGO) quenched peptide probe, we constructed a new biosensing system for monitoring in vitro and intracellular caspase-8 activity. In this system, a fluorophore-labeled peptide and rGO were used as the substrate of caspase-8 and the fluorophore quencher, respectively. The hydrolysis of caspase-8 on the polypeptide probe substrate can generate two fragments with different lengths. The release of the short fragment labeled with the fluorophore causes recovery of the fluorescence signal (Ex/Em = 520/576 nm). Under the optimized conditions, the proposed fluorescence method exhibited a linear response range of 0.2 to 5 U·mL−1 for caspase-8 with a limit of detection (LOD) of 0.2 U·mL−1 in vitro. Furthermore, this method has been successfully applied to monitoring the upregulation of intracellular caspase-8 activity caused by tert-butyl hydroperoxide (TBHP) and fluorouracil. Flow cytometry assay indicated the positive relation between the upregulation of intracellular caspase-8 activity and cell apoptosis rate. In summary, the above results demonstrated the practical application of this method for apoptosis-related cell imaging.
Graphical Abstract









Similar content being viewed by others
References
Cheng X, Ferrell JJ (2018) Apoptosis propagates through the cytoplasm as trigger waves. Science 361(6402):607–612
Jost PJ, Grabow S, Gray D et al (2009) XIAP discriminates between type I and type II FAS-induced apoptosis. Nature 460(7258):1035–1039
Fritsch M, Gunther SD, Schwarzer R et al (2019) Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575(7784):683–687
Newton K, Wickliffe KE, Dugger DL et al (2019) Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature 574(7778):428–431
Carneiro BA, El-Deiry WS (2020) Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol 17(7):395–417
Wang H, Yu Q, Zhang Z et al (2020) Involvement of the miR-137-3p/CAPN-2 interaction in ischemia-reperfusion-induced neuronal apoptosis through modulation of p35 cleavage and subsequent caspase-8 overactivation. Oxid Med Cell Longev 2020:2616871
Boege Y, Malehmir M, Healy ME et al (2017) A dual role of caspase-8 in triggering and sensing proliferation-associated DNA damage, a key determinant of liver cancer development. Cancer Cell 32(3):342–359
Benkova B, Lozanov V, Ivanov IP et al (2009) Evaluation of recombinant caspase specificity by competitive substrates. Anal Biochem 394(1):68–74
Su J, Rajapaksha TW, Peter ME et al (2006) Assays of endogenous caspase activities: a comparison of mass spectrometry and fluorescence format. Anal Chem 78(14):4945–4951
den Hamer A, Dierickx P, Arts R et al (2017) Bright bioluminescent BRET sensor proteins for measuring intracellular caspase activity. ACS Sens 2(6):729–734
Zeng Y, Du Q, Zhang Z et al (2020) Curcumin promotes cancer-associated fibroblasts apoptosis via ROS-mediated endoplasmic reticulum stress. Arch Biochem Biophys 694:108613
Corbat AA, Schuermann KC, Liguzinski P et al (2018) Co-imaging extrinsic, intrinsic and effector caspase activity by fluorescence anisotropy microscopy. Redox Biol 19:210–217
Zhu W, Wang C, Hu J et al (2021) Promoted “Click” SERS detection for precise intracellular imaging of caspase-3. Anal Chem 93(11):4876–4883
Zhou H, Luo R, Xie Q et al (2021) A new fluorescence method for monitoring PNK activity in vitro, natural compounds screening and intracellular imaging. Sens Actuators B 329:129203
Tong C, Zhou T, Zhao C et al (2019) Fluorometric determination of RNase H via a DNAzyme conjugated to reduced graphene oxide, and its application to screening for inhibitors and activators. Microchim Acta 186(6):335
Qiu Y, Dang W, Fan J et al (2020) DNAzyme and rGO based fluorescence assay for Fpg activity analysis, drug screening, and bacterial imaging. Talanta 218:121158
Liu M, Zhang D, Zhang X et al (2020) Label-free and amplified detection of apoptosis-associated caspase activity using branched rolling circle amplification. Chem Commun 56(39):5243–5246
Liu X, Song X, Luan D et al (2019) Real-time in situ visualizing of the sequential activation of caspase cascade using a multicolor gold–selenium bonding fluorescent nanoprobe. Anal Chem 91(9):5994–6002
Kwon OS, Song HS, Park TH et al (2019) Conducting nanomaterial sensor using natural receptors. Chem Rev 119(1):36–93
Tong C, Zhao C, Liu B et al (2018) Sensitive detection of RNase A activity and collaborative drug screening based on rGO and fluorescence probe. Anal Chem 90(4):2655–2661
Luo R, Zhou H, Dang W et al (2020) A DNAzyme-rGO coupled fluorescence assay for T4PNK activity in vitro and intracellular imaging. Sens Actuators B 310:127884
Dang W, Liu H, Fan J et al (2019) Monitoring VEGF mRNA and imaging in living cells in vitro using rGO-based dual fluorescent signal amplification platform. Talanta 205:120092
Qin Y, Fan J, Yang W et al (2020) Endogenous Cys-assisted GSH@AgNCs-rGO nanoprobe for real-time monitoring of dynamic change in GSH levels regulated by natural drug. Anal Chem 92(2):1988–1996
Blanchard H, Donepudi M, Tschopp M et al (2000) Caspase-8 specificity probed at subsite S(4): crystal structure of the caspase-8-Z-DEVD-cho complex. J Mol Biol 302(1):9–16
Xie Y, Zhao R, Tan Y et al (2012) Conjugated polymer-based real-time fluorescence caspase assays. ACS Appl Mater Interfaces 4(1):405–410
Liu W, Liu S, Kuang Y et al (2016) Developing activity localization fluorescence peptide probe using thiol-ene click reaction for spatially resolved imaging of caspase-8 in live cells. Anal Chem 88(15):7867–7872
Zhang L, Xu H, Xia Y et al (2021) Real-time monitoring of caspase-3/8 activity by self-assembling nanofiber probes in living cells. Chem Commun 57(6):797–800
Yuan Y, Zhang C, Kwok R et al (2017) Light-up probe based on AIEgens: dual signal turn-on for caspase cascade activation monitoring. Chem Sci 8:2723–2728
Pirnia F, Schneider E, Betticher D et al (2002) Mitomycin C induces apoptosis and caspase-8 and -9 processing through a caspase-3 and Fas-independent pathway. Cell Death Differ 9(9):905–914
Zhou T, Luo R, Li Y et al (2020) Activity assay and intracellular imaging of APE1 assisted with tetrahedral DNA nanostructure modified-dnazyme and molecular beacon. Sens Actuators B 317:128203
Xiao X, Guo M, Li Q et al (2008) In-situ monitoring of breast cancer cell (MCF-7) growth and quantification of the cytotoxicity of anticancer drugs fluorouracil and cisplatin. Biosens Bioelectron 24(2):247–252
Fedoreyeva L, Kireev I, Khavinson V et al (2011) Penetration of short fluorescence-labeled peptides into the nucleus in HeLa cells and in vitro specific interaction of the peptides with deoxyribooligonucleotides and DNA. Biochemistry Moscow 76:1210–1219
Funding
This work was partially supported by the Natural Science Foundation of Hunan Province (2021JJ30164), the National Standardization Project of Traditional Chinese Medicine (ZYBZH-Y-HUN-23), Key Research and Development Program of Hainan Province (ZDYF2022SHFS075), Science and Technology Innovation Leading Plan for High-tech Industry of Hunan Province (2020SK2043).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tao, X., Zou, W., Qin, Y. et al. Reduced graphene oxide quenched peptide probe for caspase-8 activity detection and cellular imaging. Microchim Acta 189, 463 (2022). https://doi.org/10.1007/s00604-022-05567-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05567-0