Abstract
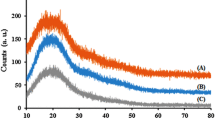
A novel hydrophilic As(V) ion-imprinted cryogel (IIC) was green prepared by cryogelation in aqueous environment which was coincident with the adsorption condition and can improve the specific recognition performance. The methacrylamido propyl trimethyl ammonium chloride (MPTAC) was selected as the functional monomer and the saturated adsorption capacity of the prepared IIC and NIC were 55.0 mg/g and 29.4 mg/g, and with high imprinting factor of 1.87. Additionally, the prepared IIC showed admirable reusability and high selectivity, and the recovery was in the range 81.2–97.9% with RSD range of 1.9–4.3%, which was similar to the value obtained by hydride generation atomic absorption spectrometry. IIC can be used as solid material for colorimetric detection at the ultraviolet wavelength of 858 nm without color interference of material matrix, in the range 5–200 μg/L (R2 = 0.990) with a detection limit of 0.970 µg/L. Obviously, this synthetic strategy provides a simple, efficient, and green method for the preparation of water-compatible ion-imprinted polymers providing selective separation and detection of trace As(V) in real complex samples.
Graphical Abstract







Similar content being viewed by others
Change history
04 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00604-023-05695-1
References
Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodes CJ, Valko M (2011) Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 31:95–107. https://doi.org/10.1002/jat.1649
Minatel BC, Sage AP, Anderson C, Hubaux R, Marshall EA, Lam WL, Martinez VD (2018) Environmental arsenic exposure: from genetic susceptibility to pathogenesis. Environ Int 112:183–197. https://doi.org/10.1016/j.envint.2017.12.017
Urbano BF, Villenas I, Rivas BL, Campos CH (2015) Cationic polymer–TiO2 nanocomposite sorbent for arsenate removal. Chem Eng J 268:362–370. https://doi.org/10.1016/j.cej.2015.01.068
Deng S, Zhang G, Chen S, Xue Y, Du Z, Wang P (2016) Rapid and effective preparation of a HPEI modified biosorbent based on cellulose fiber with a microwave irradiation method for enhanced arsenic removal in water. J Mater Chem A 4:15851–15860. https://doi.org/10.1039/c6ta06051j
Zuo JC, Tong SR, Yu XL, Wu LY, Cao CY, Ge MF, Song WG (2012) Fe3+ and amino functioned mesoporous silica: preparation, structural analysis and arsenic adsorption. J Hazard Mater 235–236:336–342. https://doi.org/10.1016/j.jhazmat.2012.08.009
Zhang M, Ma X, Li J, Huang R, Guo L, Zhang X, Fan Y, Xie X, Zeng G (2019) Enhanced removal of As() and As() from aqueous solution using ionic liquid-modified magnetic graphene oxide. Chemosphere 234:196–203. https://doi.org/10.1016/j.chemosphere.2019.06.057
Pramanik K, Sarkar P, Bhattacharyay D (2019) 3Mercaptopropanoic acid modified cellulose filter paper for quick removal of arsenate from drinking water. Int J Biol Macromol 122:185–194. https://doi.org/10.1016/j.ijbiomac.2018.10.065
Hokkanen S, Repo E, Lou S, Sillanpää M (2015) Removal of arsenic(V) by magnetic nanoparticle activated microfibrillated cellulose. Chem Eng J 260:886–894. https://doi.org/10.1016/j.cej.2014.08.093
Kyzas GZ, Siafaka PI, Kostoglou M, Bikiaris DN (2016) Adsorption of As(III) and As(V) onto colloidal microparticles of commercial cross-linked polyallylamine (Sevelamer) from single and binary ion solutions. J Colloid Interface Sci 474:137–145. https://doi.org/10.1016/j.jcis.2016.04.027
Huang Y, Wang R (2018) Review on Fundamentals, Preparations and Applications of Imprinted Polymers. Curr Org Chem 22:1600–1618. https://doi.org/10.2174/1385272822666180711120045
Fu J, Chen L, Li J, Zhang Z (2015) Current status and challenges of ion imprinting. J Mater Chem A 3:13598–13627. https://doi.org/10.1039/c5ta02421h
Lu J, Qin Y, Wu Y, Meng M, Yan Y, Li C (2019) Recent advances in ion-imprinted membranes: separation and detection via ion-selective recognition. Environ Sci Water Res Technol 5:1626–1653. https://doi.org/10.1039/c9ew00465c
Shakerian F, Kim K-H, Kwon E, Szulejko JE, Kumar P, Dadfarnia S, Haji Shabani AM (2016) Advanced polymeric materials: synthesis and analytical application of ion imprinted polymers as selective sorbents for solid phase extraction of metal ions. Trends Anal Chem 83:55–69. https://doi.org/10.1016/j.trac.2016.08.001
Zhao S, Zou Y, Liu X, Zhang H (2019) Ecofriendly construction of enzyme reactor based on three-dimensional porous cryogel composites. Chem Eng J 361:286–293. https://doi.org/10.1016/j.cej.2018.12.101
Wang C, Dong X-Y, Jiang Z, Sun Y (2013) Enhanced adsorption capacity of cryogel bed by incorporating polymeric resin particles. J Chromatogr A 1272:20–25. https://doi.org/10.1016/j.chroma.2012.11.059
Hedström M, Plieva F, Galaev IY, Mattiasson B (2008) Monolithic macroporous albumin/chitosan cryogel structure: a new matrix for enzyme immobilization. Anal Bioanal Chem 390:907–912. https://doi.org/10.1007/s00216-007-1745-6
Koç İ, Baydemir G, Bayram E, Yavuz H, Denizli A (2011) Selective removal of 17β-estradiol with molecularly imprinted particle-embedded cryogel systems. J Hazard Mater 192:1819–1826. https://doi.org/10.1016/j.jhazmat.2011.07.017
Chen M, Lin Y, Gu C, Wang J (2013) Arsenic sorption and speciation with branch-polyethyleneimine modified carbon nanotubes with detection by atomic fluorescence spectrometry. Talanta 104:53–57. https://doi.org/10.1016/j.talanta.2012.11.034
Boyaci E, Cagir A, Shahwan T, Eroglu AE (2011) Synthesis, characterization and application of a novel mercapto- and amine-bifunctionalized silica for speciation/sorption of inorganic arsenic prior to inductively coupled plasma mass spectrometric determination. Talanta 85:1517–1525. https://doi.org/10.1016/j.talanta.2011.06.021
Devi P, Thakur A, Lai RY, Saini S, Jain R, Kumar P (2019) Progress in the materials for optical detection of arsenic in water. TrAC, Trends Anal Chem 110:97–115. https://doi.org/10.1016/j.trac.2018.10.008
Hu S, Lu J, Jing C (2012) A novel colorimetric method for field arsenic speciation analysis. J Environ Sci 24:1341–1346. https://doi.org/10.1016/s1001-0742(11)60922-4
Saiz J, Bringas E, Ortiz I (2014) New functionalized magnetic materials for As5+ removal: adsorbent regeneration and reuse. Ind Eng Chem Res 53:18928–18934. https://doi.org/10.1021/ie500912k
Ma J, Sengupta MK, Yuan D, Dasgupta PK (2014) Speciation and detection of arsenic in aqueous samples: a review of recent progress in non-atomic spectrometric methods. Anal Chim Acta 831:1–23. https://doi.org/10.1016/j.aca.2014.04.029
Okazaki T, Kuramitz H, Hata N, Taguchi S, Murai K, Okauchi K (2015) Visual colorimetry for determination of trace arsenic in groundwater based on improved molybdenum blue spectrophotometry. Anal Methods 7:2794–2799. https://doi.org/10.1039/c4ay03021d
Lenoble V (2003) Arsenite oxidation and arsenate determination by the molybdene blue method. Talanta 61:267–276. https://doi.org/10.1016/s0039-9140(03)00274-1
Wang M, Qiao F, Yan H (2021) A simple and benign protocol for the synthesis of a deep eutectic solvent-based hydrophilic molecularly imprinted resin in water for excellent selective molecular recognition in aqueous phase. Green Chem. https://doi.org/10.1039/d1gc00789k
Liang S, Yan H, Cao J, Han Y, Shen S, Bai L (2017) Molecularly imprinted phloroglucinol-formaldehyde-melamine resin prepared in a deep eutectic solvent for selective recognition of clorprenaline and bambuterol in urine. Anal Chim Acta 951:68–77. https://doi.org/10.1016/j.aca.2016.11.009
Najib N, Christodoulatos C (2019) Removal of arsenic using functionalized cellulose nanofibrils from aqueous solutions. J Hazard Mater 367:256–266. https://doi.org/10.1016/j.jhazmat.2018.12.067
Liu L, Yang Z, Zhao L, Liu J, Liu X, Xue J, Tang A (2020) Removal performance and mechanism of poly(N(1), N(1), N(3), N(3)-tetraallylpropane-1,3-diaminium chloride) toward Cr(VI). Environ Technol 41:2450–2463. https://doi.org/10.1080/09593330.2019.1567825
Ray J, Jana S, Tripathy T (2018) Synthesis of dipolar grafted hydroxyethyl cellulose and its application for the removal of phosphate ion from aqueous medium by adsorption. Int J Biol Macromol 109:492–506. https://doi.org/10.1016/j.ijbiomac.2017.12.083
Hamza MF, Lu S, Salih KAM, Mira H, Dhmees AS, Fujita T, Wei Y, Vincent T, Guibal E (2020) As(V) sorption from aqueous solutions using quaternized algal/polyethyleneimine composite beads. Sci Total Environ 719:137396. https://doi.org/10.1016/j.scitotenv.2020.137396
Xu X, Lin L, Papelis C, Myint M, Cath TY, Xu P (2015) Use of drinking water treatment solids for arsenate removal from desalination concentrate. J Colloid Interface Sci 445:252–261. https://doi.org/10.1016/j.jcis.2014.12.090
Sun XF, Ma Y, Liu XW, Wang SG, Gao BY, Li XM (2010) Sorption and detoxification of chromium(VI) by aerobic granules functionalized with polyethylenimine. Water Res 44:2517–2524. https://doi.org/10.1016/j.watres.2010.01.027
Manna J, Shilpa N, Bandarapu AK, Rana RK (2019) Oxyanion-binding in a bioinspired nanoparticle-assembled hybrid microsphere structure: effective removal of arsenate/chromate from water. ACS Appl Nano Mater 2:1525–1532. https://doi.org/10.1021/acsanm.9b00003
Matsunaga H, Kanno C, Suzuki TM (2005) Naked-eye detection of trace arsenic(V) in aqueous media using molybdenum-loaded chelating resin having beta-hydroxypropyl-di(beta-hydroxyethyl)amino moiety. Talanta 66:1287–1293. https://doi.org/10.1016/j.talanta.2005.01.057
Funding
The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (Grant No. 31671934) and the Science and Technology Commission of Shanghai Municipality (Grant No. 20392001600).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In this article the graphics relating to Figs. 3 and 4 images had been replaced incorrectly; the figure(s) should have appeared as shown below.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yin, F., Yang, H., Liu, X. et al. Aqueous phase synthesis of ion-imprinted cryogel for paper-based colorimetric detection of As(V) with high selectivity. Microchim Acta 190, 35 (2023). https://doi.org/10.1007/s00604-022-05564-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05564-3