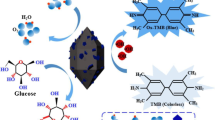
Abstract
Peroxidase mimicking Fe3O4@Chitosan (Fe3O4@Chi) nanozyme was synthesized and used for high-sensitive enzyme-free colorimetric detection of H2O2. The nanozyme was characterized in comparison with Fe3O4 nanoparticles (NPs) using X-ray diffraction, Fourier-transform infrared spectroscopy, dynamic light scattering, and thermogravimetric analysis. The catalytic performance of Fe3O4@Chi nanozyme was first evaluated by UV–Vis spectroscopy using 3,3′,5,5′-tetramethylbenzidine. Unlike Fe3O4NPs, Fe3O4@Chi nanozyme exhibited an intrinsic peroxidase activity with a detection limit of 69 nM. Next, the nanozyme was applied to a microfluidic paper-based analytical device (µPAD) and colorimetric analysis was performed at varying concentrations of H2O2 using a machine learning-based smartphone app called “Hi-perox Sens++ .” The app with machine learning classifiers made the system user-friendly as well as more robust and adaptive against variation in illumination and camera optics. In order to train various machine learning classifiers, the images of the µPADs were taken at 30 s and 10 min by four smartphone brands under seven different illuminations. According to the results, linear discriminant analysis exhibited the highest classification accuracy (98.7%) with phone-independent repeatability at t = 30 s and the accuracy was preserved for 10 min. The proposed system also showed excellent selectivity in the presence of various interfering molecules and good detection performance in tap water.
Graphical abstract







Similar content being viewed by others
References
Ostovan A, Arabi M, Wang Y, Li J, Li B, Wang X, Chen L (2022) Greenificated molecularly imprinted materials for advanced applications. Adv Mater. https://doi.org/10.1002/adma.202203154
Chakraborty A, Acharya H (2021) Magnetically separable Fe3O4 NPs/MIL-53 (Al) nanocomposite catalyst for intrinsic OPD oxidation and colorimetric hydrogen peroxide detection. Colloids Surf A Physicochem Eng Asp 624:126830
Liang M, Yan X (2019) Nanozymes: from new concepts, mechanisms, and standards to applications. Acc Chem Res 52:2190–2200
Kilic B, Dogan V, Kilic V, Kahyaoglu LN (2022) Colorimetric food spoilage monitoring with carbon dot and UV light reinforced fish gelatin films using a smartphone application. Int J Biol Macromole 209:1562–1572
Guascito M, Filippo E, Malitesta C, Manno D, Serra A, Turco A (2008) A new amperometric nanostructured sensor for the analytical determination of hydrogen peroxide. Biosens Bioelectron 24:1057–1063
Zhang X, Bi X, Di W, Qin W (2016) A simple and sensitive Ce(OH)CO3/H2O2/TMB reaction system for colorimetric determination of H2O2 and glucose. Sens Actuators B Chem 231:714–722
Liu Q, Zhu R, Du H, Li H, Yang Y, Jia Q, Bian B (2014) Higher catalytic activity of porphyrin functionalized Co3O4 nanostructures for visual and colorimetric detection of H2O2 and glucose. Mater Sci Eng: C 43:321–329
Arabi M, Ostovan A, Li J, Wang X, Zhang Z, Choo J, Chen L (2021) Molecular imprinting: green perspectives and strategies. Adv Mater 33:2100543
Golcez T, Kilic V, Sen M (2021) A portable smartphone-based platform with an offline image processing tool for rapid paper-based colorimetric detection of glucose in artificial saliva. Anal Sci 37:561–567
Arabi M, Chen L (2022) Technical challenges of molecular-imprinting-based optical sensors for environmental pollutants, Langmuir 38:5963–5967
Mercan ÖB (2020) Deep learning based colorimetric classification of glucose with au-ag nanoparticles using smartphone, Medical Technologies Congress (TIPTEKNO), IEEE, pp 1–4
Doğan V, Yüzer E, Kılıç V, Şen M (2021) Non-enzymatic colorimetric detection of hydrogen peroxide using a μPAD coupled with a machine learning-based smartphone app. Analyst 146:7336–7344
Mercan ÖB, Kılıç V, Şen M (2021) Machine learning-based colorimetric determination of glucose in artificial saliva with different reagents using a smartphone coupled μPAD. Sen Actuators B Chem 329:129037
Li G-y, Jiang Y-r, Huang K-l, Ding P, Chen J (2008) Preparation and properties of magnetic Fe3O4–chitosan nanoparticles. J Alloys Compd 466:451–456
Ragavan K, Ahmed SR, Weng X, Neethirajan S (2018) Chitosan as a peroxidase mimic: paper based sensor for the detection of hydrogen peroxide. Sens Actuators B Chem 272:8–13
Akyazi T, Basabe-Desmonts L, Benito-Lopez F (2018) Review on microfluidic paper-based analytical devices towards commercialisation. Anal Chim Acta 1001:1–17
Martinez AW, Phillips ST, Butte MJ, Whitesides GM (2007) Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew Chem Int Ed 119:1340–1342
Kiliç V, Mercan ÖB, Tetik M, Kap Ö, Horzum N (2022) Non-enzymatic colorimetric glucose detection based on Au/Ag nanoparticles using smartphone and machine learning. Anal Sci 38: 347–358
Mercan ÖB, Doğan V, Kılıç V (2020) Time series analysis based machine learning classification for blood sugar levels, Medical Technologies Congress (TIPTEKNO), IEEE, pp 1–4
Srivastava M, Singh J, Yashpal M, Gupta DK, Mishra R, Tripathi S, Ojha AK (2012) Synthesis of superparamagnetic bare Fe3O4 nanostructures and core/shell (Fe3O4/alginate) nanocomposites. Carbohydr Polym 89:821–829
Mohammadi A, Barikani M (2014) Synthesis and characterization of superparamagnetic Fe3O4 nanoparticles coated with thiodiglycol. Mater Charact 90:88–93
Li S, Zhang T, Tang R, Qiu H, Wang C, Zhou Z (2015) Solvothermal synthesis and characterization of monodisperse superparamagnetic iron oxide nanoparticles. J Magn Magn Mater 379:226–231
Cao S-L, Li X-H, Lou W-Y, Zong M-H (2014) Preparation of a novel magnetic cellulose nanocrystal and its efficient use for enzyme immobilization. J Mater Chem B 2:5522–5530
Mucha M, Pawlak A (2002) Complex study on chitosan degradability. Polim 47:509–516
Fan Z, Xu Y, Zhang D (2011) Local linear discriminant analysis framework using sample neighbors. IEEE Trans Neural Netw 22:1119–1132
Tharwat A, Gaber T, Ibrahim A, Hassanien AE (2017) Linear discriminant analysis: A detailed tutorial. AI Commun 30:169–190
Chicco D, Jurman G (2020) The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genomics 21:1–13
Manimurugan S, Al-Mutairi S, Aborokbah MM, Chilamkurti N, Ganesan S, Patan R (2020) Effective attack detection in internet of medical things smart environment using a deep belief neural network. IEEE Access 8:77396–77404
Verma S, Choudhary J, Singh KP, Chandra P, Singh SP (2019) Uricase grafted nanoconducting matrix based electrochemical biosensor for ultrafast uric acid detection in human serum samples. Int J Biol Macromole 130:333–341
Guo X, Wang Y, Wu F, Ni Y, Kokot S (2015) A colorimetric method of analysis for trace amounts of hydrogen peroxide with the use of the nano-properties of molybdenum disulfide. Analyst 140:1119–1126
Riaz MA, Chen Y (2022) Electrodes and electrocatalysts for electrochemical hydrogen peroxide sensors: a review of design strategies, Nanoscale Horiz 7:463-479
Ahmed SR, Cirone J, Chen A (2019) Fluorescent Fe3O4 quantum dots for H2O2 detection. ACS Appl Nano Mater 2:2076–2085
Yousefinejad S, Rasti H, Hajebi M, Kowsari M, Sadravi S, Honarasa F (2017) Design of C-dots/Fe3O4 magnetic nanocomposite as an efficient new nanozyme and its application for determination of H2O2 in nanomolar level. Sens Actuators B Chem 247:691–696
Su L, Cai Y, Wang L, Dong W, Mao G, Li Y, Zhao M, Ma Y, Zhang H (2020) Hemin@carbon dot hybrid nanozymes with peroxidase mimicking properties for dual (colorimetric and fluorometric) sensing of hydrogen peroxide, glucose and xanthine. Microchim Acta 187:1–11
Zhang W, Li X, Cui T, Li S, Qian Y, Yue Y, Zhong W, Xu B, Yue W (2021) PtS2 nanosheets as a peroxidase-mimicking nanozyme for colorimetric determination of hydrogen peroxide and glucose. Microchim Acta 188:1–9
Xian J, Weng Y, Guo H, Li Y, Yao B, Weng W (2019) One-pot fabrication of Fe-doped carbon nitride nanoparticles as peroxidase mimetics for H2O2 and glucose detection. Spectrochimica Acta Part A Mole Biomol Spectrosc 215:218–224
Liu T, Tian J, Cui L, Liu Q, Wu L, Zhang X (2019) Facile strategy to prepare a metalloporphyrin-based hydrophilic porous organic polymer with enhanced peroxidase-like activity and high stability for colorimetric detection of H2O2 and glucose. Colloids Surf B Biointerfaces 178:137–145
Sengupta P, Pramanik K, Datta P, Sarkar P (2020) Chemically modified carbon nitride-chitin-acetic acid hybrid as a metal-free bifunctional nanozyme cascade of glucose oxidase-peroxidase for “click off” colorimetric detection of peroxide and glucose. Biosens Bioelectron 154:112072
Ngo Y-LT, Nguyen PL, Jana J, Choi WM, Chung JS, Hur SH (2021) Simple paper-based colorimetric and fluorescent glucose sensor using N-doped carbon dots and metal oxide hybrid structures. Anal Chim Acta 1147:187–198
Cheng D, Qin J, Feng Y, Wei J (2021) Synthesis of mesoporous CuO hollow sphere nanozyme for paper-based hydrogen peroxide sensor. Biosens 11:258
Funding
This research was supported by the scientific research projects coordination unit of Izmir Katip Celebi University (Project Nos. 2021-GAP-MÜMF-0054, 2022-ÖDL-MÜMF-0007, and 2021-GAP-MÜMF-0055).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Şen, M., Yüzer, E., Doğan, V. et al. Colorimetric detection of H2O2 with Fe3O4@Chi nanozyme modified µPADs using artificial intelligence. Microchim Acta 189, 373 (2022). https://doi.org/10.1007/s00604-022-05474-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05474-4