Abstract
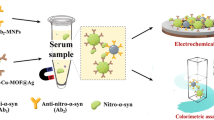
A self-assembled nanozyme of iron porphyrin mediated supramolecular modified gold nanoparticles (FpA) was fabricated to determine nitrated alpha-synuclein as the Tyr 39 residue (nT39 α-Syn) of a potential biomarker for early diagnosis of Parkinson’s disease (PD). Mechanically, localized surface plasmon resonance (LSPR) and the mass effect caused by catalytic deposition of the nanozyme contributed to a cascade signal amplification strategy. The sensor allowed a signal amplification and selective nT39 α-Syn bioanalysis with a 1.34-fold enhancement by cascade amplified SPR signal and double specific recognition. The detection limit was 1.78 ng/mL in the detection range of 7–240 ng/mL. Benefiting from the excellent immunosensor, this method can distinguish healthy people and PD patients using actual samples. Overall, this strategy provides a nanozyme-based biosensing platform for the early diagnosis of PD and can be applied to detect other protein biomarkers, such as PD-L1.
Graphical abstract







Similar content being viewed by others
References
Ball N, Teo WP, Chandra S, Chapman J (2019) Parkinson’s disease and the environment. Front Neurol 10:218
Dawson TM, Dawson VL (2003) Molecular pathways of neurodegeneration in Parkinson’s disease. Science 302(5646):819–822
Tysnes OB, Storstein A (2017) Epidemiology of Parkinson’s disease. J Neural Transm 124(8):901–905
Bridi JC, Frank H (2018) Mechanisms of α-synuclein induced synaptopathy in Parkinson’s disease. Front Neurosci 12:80
Burai R, Ait-Bouziad N, Chiki A, Lashuel HA (2015) Elucidating the role of site-specific nitration of α-synuclein in the pathogenesis of Parkinson’s disease via protein semisynthesis and mutagenesis. J Am Chem Soc 137(15):5041–5052
Desplats P, Lee HJ, Bae EJ, Patrick C, Lee SJ (2009) Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc Natl Acad Sci 106(31):13010–13015
Tao D, Gu Y, Song S, Nguyen EP, Cheng J, Yuan Q, Pan H, Jaffrezic N, Guo Z (2020) Ultrasensitive detection of alpha-synuclein oligomer using a PolyD-glucosamine/gold nanoparticle/carbon-based nanomaterials modified electrochemical immunosensor in human plasma. Microchem J 158:105195
Ma LY, Gao LY, Li X, Ma HZ, Feng T (2019) Nitrated alpha-synuclein in minor salivary gland biopsies in Parkinson’s disease. Neurosci Lett 704:45–49
Qiao HH, Zhu LN, Wang Y, Hui JL, Xie WB (2019) Implications of alpha-synuclein nitration at tyrosine 39 in methamphetamine-induced neurotoxicity in vitro and in vivo. Neural Regener Res 14(2):319
Zhang ZH, Hu J, Chen Q, Chen J, Hu X, Koh K, Chen H, Xu XH (2021) The magnetic-nanoparticle-assisted sensitive detection of nitrated α-syn in blood based on a sensitizing electrochemical layer. Nanoscale 13(17):8107–8117
Ma Y, Hu Q, Liu C, Wang L (2020) A nanospherical conjugated microporous polymer-graphene nanosheets modified molecularly imprinted electrochemical sensor for high sensitivity detection of α-synuclein. J Electroanal Chem 862:113994
Sun K, Xia N, Zhao L, Liu K, Hou W, Liu L (2017) Aptasensors for the selective detection of alpha-synuclein oligomer by colorimetry, surface plasmon resonance and electrochemical impedance spectroscopy. Sens Actuators B-Chem 245:87–94
Ertürk G, Özen H, Tümer MA, Mattiasson B, Denizli A (2016) Microcontact imprinting based surface plasmon resonance (SPR) biosensor for real-time and ultrasensitive detection of prostate specific antigen (PSA) from clinical samples. Sens Actuators B-Chem 224:823–832
Koyun S, Akgnüllü S, Yavuz H, Erdem A, Denizli A (2019) Surface plasmon resonance aptasensor for detection of human activated protein C. Talanta 194:528–533
Piliarik M, Vaisocherová H, Homola J (2005) A new surface plasmon resonance sensor for high-throughput screening applications. Biosens Bioelectron 20(10):2104–2110
Vu Nu TT, Tran N, Nam E, Nguyen TT, Yoon WJ, Cho S, Kim J, Chang KA, Ju H (2018) Blood-based immunoassay of tau proteins for early diagnosis of Alzheimer’s disease using surface plasmon resonance fiber sensors. RSC Adv 8(14):7855–7862
Chang L, Xie Z, An Z, Yang Y, Maxwell E, Gu X, Miquel JJ, Dy GK, Mary R, Gan Q (2018) Sensitive detection of exosomal proteins via a compact surface plasmon resonance biosensor for cancer diagnosis. Acs Sens 3(8):1471–1479
Kim DH, Cho IH, Park JN, Paek SH, Paek SH (2017) Semi-continuous real-time monitoring of protein biomarker using a recyclable surface plasmon resonance sensor. Biosens Bioelectron 88:232–239
Sheh O, Wing FY, Jaafar A, Engku C, Adzir MM (2018) Development of an optical sensor based on surface plasmon resonance phenomenon for diagnosis of dengue virus E -protein. Sens Biosensing Res 20:16–21
Li Q, Wang Q, Yang X, Wang K, Zhang H, Nie W (2017) High sensitivity surface plasmon resonance biosensor for detection of microRNA and small molecule based on graphene oxide-gold nanoparticles composites. Talanta 174:521
Farzaneh F, Rashidi MR, Yadollah O (2019) Ultra-sensitive detection by metal nanoparticles-mediated enhanced SPR biosensors. Talanta 192:118–127
Li J, Lei P, Ding S, Zhang Y, Yang J, Cheng Q, Yan Y (2016) An enzyme-free surface plasmon resonance biosensor for real-time detecting microRNA based on allosteric effect of mismatched catalytic hairpin assembly. Biosens Bioelectron 77:435–441
Yao GH, Liang RP, Yu XD, Huang CF, Zhang L, Qiu JD (2015) Target-triggering multiple-cycle amplification strategy for ultrasensitive detection of adenosine based on surface plasma resonance techniques. Anal Chem 87(2):929–936
Li X, Cheng W, Li D, Wu J, Ding X, Cheng Q, Ding S (2016) A novel surface plasmon resonance biosensor for enzyme-free and highly sensitive detection of microRNA based on multi component nucleic acid enzyme (MNAzyme)-mediated catalyzed hairpin assembly. Biosens Bioelectron 80:98–104
Miyazaki CM, Shimizu FM, Mejía-Salazar J, Oliveira ON, Ferreira M (2017) Surface plasmon resonance biosensor for enzymatic detection of small analytes. Nanotechnol 28(14):145501
Zdeněk F, Juřík T, Matěj P, Skládal P (2016) Enzymatic precipitation enhanced surface plasmon resonance immunosensor for the detection of salmonella in powdered milk. Anal Chem 88(23):11830–11836
Su X, O’Shea SJ (2001) Determination of monoenzyme- and bienzyme-stimulated precipitation by a cuvette-based surface plasmon resonance instrument. Anal Biochem 299(2):241–246
Zhang C, Zhang D, Ma Z, Han H (2019) Cascade catalysis-initiated radical polymerization amplified impedimetric immunosensor for ultrasensitive detection of carbohydrate antigen 15–3. Biosens Bioelectron 137:1–7
Xu L, Liu Z, Lei S, Huang D, Ye B (2019) A sandwich-type electrochemical aptasensor for the carcinoembryonic antigen via biocatalytic precipitation amplification and by using gold nanoparticle composites. Microchim Acta 186(7):1–9
Zhang XY, Liu SG, Zhang WJ, Wang XH, Han L, Ling Y, Li NB, Luo HQ (2019) Photoelectrochemical platform for glucose sensing based on g-C3N4/ZnIn2S4 composites coupled with bi-enzyme cascade catalytic in-situ precipitation. Sens Actuators B-Chem 297:126818
Borthakur P, Boruah PK, Das MR (2021) Facile synthesis of CuS nanoparticles on two-dimensional nanosheets as efficient artificial nanozyme for detection of Ibuprofen in water. J Environ Chem Eng 9(1):104635
Cai S, Fu Z, Xiao W, Xiong Y, Wang C, Yang R (2020) Zero-dimensional/two-dimensional AuxPd100–x nanocomposites with enhanced nanozyme catalysis for sensitive glucose detection. ACS Appl Mater Interfaces 12(10):11616–11624
Fu J, Zhou Y, Huang X, Zhang W, Xiong Y (2020) Dramatically enhanced immunochromatographic assay using cascade signal amplification for ultrasensitive detection of escherichia coli O157:H7 in milk. J Agric Food Chem 68(4):1118–1125
Prasad SN, Weerathunge P, Karim MN, Anderson S, Ramanathan R (2021) Non-invasive detection of glucose in human urine using a color-generating copper NanoZyme. Anal Bioanal Chem 413(5):1279–1291
Duan D, Fan K, Zhang D, Tan S, Liang M, Liu Y, Zhang J, Zhang P, Liu W, Qiu X (2015) Nanozyme-strip for rapid local diagnosis of Ebola. Biosens Bioelectron 74:134–141
Yuan PX, Deng SY, Xin P, Ji XB, Shan D, Cosnier S (2015) Mass effect of redox reactions: a novel mode for surface plasmon resonance-based bioanalysis. Biosens Bioelectron 74:183–189
Hu X, Chen J, Hu R, Zhu Z, Lai Z, Zhu X, Zhu H, Koh K, Chen H (2021) Synergistically catalytic nanozymes based on heme-protein active site model for dual-signal and ultrasensitive detection of H2O2 in living cells. Sens Actuators B-Chem 333:129564
Fetouhi A, Benatallah L, Nawrocka A, Monika S, Bouasla A, Marta T, Zidoune M, Sujak A (2019) Investigation of viscoelastic behaviour of rice-field bean gluten-free dough using the biophysical characterization of proteins and starch: a FT-IR study. J Food Sci Technol 56:1316–1327
Arulaabaranam K, Muthu S, Mani G, Irfande A (2021) Conformational study, FT-IR, FT-Raman, solvent effect on UV–Vis, charge transfer and protein–ligand interactions of Methyl-2-pyrazinecarboxylate. J Mol Liq 341:116934
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 62005156 and 61875114).
Author information
Authors and Affiliations
Contributions
Xiaojun Hu: Conceptualization, formal analysis, and writing—original draft. Ruhui Hu: Formal analysis, validation, and writing—review and editing. Han Zhu: Formal analysis, validation, and writing—original draft. Qiang Chen: Validation, writing—original draft. Yongkai Lu: Formal analysis, writing—review and editing. Jie Chen: Data curation and writing–review and editing. Yawen Liu: Data curation and writing—review and editing. Hongxia Chen: Conceptualization, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, X., Hu, R., Zhu, H. et al. Nanozyme-based cascade SPR signal amplification for immunosensing of nitrated alpha-synuclein. Microchim Acta 189, 367 (2022). https://doi.org/10.1007/s00604-022-05465-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05465-5