Abstract
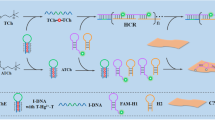
A Co, N co-doped porous carbon-based nanozyme (Co–N-C nanozyme) has been fabricated. Taking advantages of the excellent oxidase catalytic activity and significant stability of Co–N-C nanozyme, we propose a fluorescence and colorimetric system based on Co–N-C nanozyme and red-emitting carbon quantum dots (RCDs) for butyrylcholinesterase (BChE) sensing. As the chromogenic substrate 3,3′,5,5′-tetramethylbenzidine (TMB) was catalyzed and oxidized by Co–N-C nanozyme, the generated oxTMB had a new absorption peak at 652 nm, which resulted in the significant quenching of the fluorescence of the carbon quantum dots at 610 nm. Under the catalysis of BChE, thiocholine was generated from the hydrolysis of S-butyrylthiocholine iodide (BTCh), and the as-generated thiocholine effectively inhibited the oxidation of TMB catalyzed by Co–N-C nanozyme, leading to a decrease of the absorption of oxTMB at 652 nm and effective fluorescence recovery of RCDs. By measuring the absorbance of produced oxTMB at 652 nm and the fluorescence of RCDs at 610 nm, the fluorescence and colorimetric system both exhibited an outstanding linear response to the activity of BChE in the range 0.5 to 40 U L−1, with a detection limit of 0.16 U L−1 and 0.21 U L−1, respectively. Furthermore, this established dual-channel biosensing strategy has been successfully applied to the determination of BChE in human serum samples. The present work has effectively expanded the development and application of nanozyme in biosensing.
Graphical abstract






Similar content being viewed by others
References
Han X, Liu L, Gong H, Luo L, Han Y, Fan J, Xu C, Yue T, Wang J, Zhang W (2022) Dextran-stabilized Fe-Mn bimetallic oxidase-like nanozyme for total antioxidant capacity assay of fruit and vegetable food. Food Chem 371:131115. https://doi.org/10.1016/j.foodchem.2021.131115
Yang C, Jiang Z, Wu Q, Hu C, Huang C, Li Y, Zhen S (2022) One-component nano-metal-organic frameworks with superior multienzyme-mimic activities for 1,4-dihydropyridine metabolism. J Colloid Interface Sci 605:214–222. https://doi.org/10.1016/j.jcis.2021.07.107
Zhu S, Tang Y, Shi B, Zou W, Wang X, Wang C, Wu Y (2021) Oligonucleotide-mediated the oxidase-mimicking activity of Mn3O4 nanoparticles as a novel colorimetric aptasensor for ultrasensitive and selective detection of Staphylococcus aureus in food. Sensors and Actuators B: Chem 349:130809. https://doi.org/10.1016/j.snb.2021.130809
Liu T, Li Z, Chen M, Zhao H, Zheng Z, Cui L, Zhang X (2021) Sensitive electrochemical biosensor for Uracil-DNA glycosylase detection based on self-linkable hollow Mn/Ni layered doubled hydroxides as oxidase-like nanozyme for cascade signal amplification. Biosens Bioelectron 194:113607. https://doi.org/10.1016/j.bios.2021.113607
Xu Y, Li P, Hu X et al (2021) Polyoxometalate nanostructures decorated with CuO nanoparticles for sensing ascorbic acid and Fe2+ ions. ACS Appl Nano Mater 4(8):8302–8313. https://doi.org/10.1021/acsanm.1c01495
He S-B, Yang L, Lin M-T, Noreldeen H A A, Yu R-X, Peng H-P, Deng H-H, Chen W (2021) Acetaminophen sensor based on the oxidase-like activity and interference self-elimination ability of chondroitin sulfate-modified platinum nanozyme. Sensors and Actuators B: Chem 347:130627. https://doi.org/10.1016/j.snb.2021.130627
Zhao Q, Shen T, Liu Y et al (2021) Universal nanoplatform for formaldehyde detection based on the oxidase-mimicking activity of MnO2 nanosheets and the in situ catalysis-produced fluorescence species. J Agric Food Chem 69(26):7303–7312. https://doi.org/10.1021/acs.jafc.1c01174
Xu X, Ran F, Fan Z, Lai H, Cheng Z, Lv T, Shao L, Liu Y (2019) Cactus-inspired bimetallic metal-organic framework-derived 1D–2D hierarchical Co/N-decorated carbon architecture toward enhanced electromagnetic wave absorbing performance. ACS Appl Mater Interfaces 11:13564–13573. https://doi.org/10.1021/acsami.9b00356
Li X, Niu X, Liu P, Xu X, Du D, Lin Y (2020) High-performance dual-channel ratiometric colorimetric sensing of phosphate ion based on target-induced differential oxidase-like activity changes of Ce-Zr bimetal-organic frameworks. Sensors and Actuators B: Chemical 321:128546. https://doi.org/10.1016/j.snb.2020.128546
Li S, Wang L, Zhang X, Chai H, Huang Y (2018) A Co, N co-doped hierarchically porous carbon hybrid as a highly efficient oxidase mimetic for glutathione detection. Sens Actuators, B Chem 264:312–319. https://doi.org/10.1016/j.snb.2018.03.015
Liu W, Chu L, Zhang C, Ni P, Jiang Y, Wang B, Lu Y, Chen C (2021) Hemin-assisted synthesis of peroxidase-like Fe-N-C nanozymes for detection of ascorbic acid-generating bio-enzymes. Chem Eng J 415:128876. https://doi.org/10.1016/j.cej.2021.128876
Li P, Wu C, Xu Y, Cheng D, Lu Q (2020) Group IV nanodots: newly emerging properties and application in biomarkers sensing. Trend Anal Chem 131:116007. https://doi.org/10.1016/j.trac.2020.116007
Xu Y, Li P, Cheng D et al (2020) Group IV nanodots: synthesis, surface engineering and application in bioimaging and biotherapy. J Mater Chem B 8(45):10290–10308. https://doi.org/10.1039/d0tb01881c
Liu M, Mou J, Xu X, Zhang F, Xia J, Wang Z (2020) High-efficiency artificial enzyme cascade bio-platform based on MOF-derived bimetal nanocomposite for biosensing. Talanta 220:121374. https://doi.org/10.1016/j.talanta.2020.121374
Yin X, Liu P, Xu X, Pan J, Li X, Niu X (2021) Breaking the pH limitation of peroxidase-like CoFe2O4 nanozyme via vitriolization for one-step glucose detection at physiological pH. Sensors and Actuators B: Chemical 328:129033. https://doi.org/10.1016/j.snb.2020.129033
Lu N, Yan X, Gu Y, Zhang T, Liu Y, Song Y, Xu Z, Xing Y, Li X, Zhang Z, Zhai S (2021) Cobalt-decorated 3D hybrid nanozyme: a catalytic amplification platform with intrinsic oxidase-like activity. Electrochim Acta 395:139197. https://doi.org/10.1016/j.electacta.2021.139197
Yang M, Tang Q, Meng Y, Liu J, Feng T, Zhao X, Zhu S, Yu W, Yang B (2018) Reversible “Off-On” fluorescence of Zn(2+)-passivated carbon dots: mechanism and potential for the detection of EDTA and Zn(2). Langmuir 34:7767–7775. https://doi.org/10.1021/acs.langmuir.8b00947
Duan Q, Wang X, Zhang B, Li Y, Zhang W, Zhang Y, Sang S (2019) A fluorometric method for mercury(II) detection based on the use of pyrophosphate-modified carbon quantum dots. Mikrochim Acta 186:736. https://doi.org/10.1007/s00604-019-3872-0
Miao X, Qu D, Yang D, Nie B, Zhao Y, Fan H, Sun Z (2018) Synthesis of carbon dots with multiple color emission by controlled graphitization and surface functionalization. Adv Mater 30(1):1704740. https://doi.org/10.1002/adma.201704740
Liu J, Li R, Yang B (2020) Carbon dots: a new type of carbon-based nanomaterial with wide applications. ACS Cent Sci 6:2179–2195. https://doi.org/10.1021/acscentsci.0c01306
Li F, Wang X, Liu W, Wang L, Wang G (2018) One-step solvothermal synthesis of red emissive carbonized polymer dots for latent fingerprint imaging. Opt Mater 86:79–86. https://doi.org/10.1016/j.optmat.2018.09.040
Gavalas S, Kelarakis A (2021) Towards red emissive systems based on carbon dots. Nanomaterials (Basel) 11(8):2089. https://doi.org/10.3390/nano11082089
Ding H, Wei JS, Zhong N, Gao QY, Xiong HM (2017) Efficient red-emitting carbon dots with gram-scale yield for bioimaging. Langmuir 33:12635–12642. https://doi.org/10.1021/acs.langmuir.7b02385
Gao Y, Jiao Y, Lu W, Liu Y, Han H, Gong X, Xian M, Shuang S, Dong C (2018) Carbon dots with red emission as a fluorescent and colorimeteric dual-readout probe for the detection of chromium (vi) and cysteine and its logic gate operation. J Mater Chem B 6:6099–6107. https://doi.org/10.1039/c8tb01580e
Jiao Y, Gao Y, Meng Y, Lu W, Liu Y, Han H, Shuang S, Li L, Dong C (2019) One-step synthesis of label-free ratiometric fluorescence carbon dots for the detection of silver ions and glutathione and cellular imaging applications. ACS Appl Mater Interfaces 11:16822–16829. https://doi.org/10.1021/acsami.9b01319
Han Z, Wang K, Du F, Yin Z, Xie Z, Zhou S (2018) High efficiency red emission carbon dots based on phenylene diisocyanate for trichromatic white and red LEDs. J Mater Chem C 6:9631–9635. https://doi.org/10.1039/c8tc03497d
Zhu X, Pang X, Zhang Y, Yao S (2019) Titanium carbide MXenes combined with red-emitting carbon dots as a unique turn-on fluorescent nanosensor for label-free determination of glucose. J Mater Chem B 7:7729–7735. https://doi.org/10.1039/c9tb02060h
Chen G, Feng H, Jiang X, Xu J, Pan S, Qian Z (2018) Redox-controlled fluorescent nanoswitch based on reversible disulfide and its application in butyrylcholinesterase activity assay. Anal Chem 90(3):1643–1651. https://doi.org/10.1021/acs.analchem.7b02976
Guo L, Zhang YJ, Yu YL, Wang JH (2020) In situ generation of prussian blue by MIL-53 (Fe) for point-of-care testing of butyrylcholinesterase activity using a portable high-throughput photothermal device. Anal Chem 92:14806–14813. https://doi.org/10.1021/acs.analchem.0c03575
Naik RS, Liu W, Saxena A (2013) Development and validation of a simple assay for the determination of cholinesterase activity in whole blood of laboratory animals. J Appl Toxicol 33(4):290–300. https://doi.org/10.1002/jat.2730
Nechaeva N, Prokopkina T, Makhaeva G, Rudakova E, Boltneva N, Dishovsky C, Eremenko A, Kurochkin I (2018) Quantitative butyrylcholinesterase activity detection by surface-enhanced Raman spectroscopy. Sens Actuators B: Chem 259:75–82. https://doi.org/10.1016/j.snb.2017.11.174
Ding J, Qin W (2009) Potentiometric sensing of butyrylcholinesterase based on in situ generation and detection of substrates. Chem Commun (Camb): 971–973. https://doi.org/10.1039/b817064a
Chen Z, Ren X, Meng X, Tan L, Chen D, Tang F (2013) Quantum dots-based fluorescent probes for turn-on and turn-off sensing of butyrylcholinesterase. Biosens Bioelectron 44:204–209
Qu Z, Yu T, Liu Y, Bi L (2021) Determination of butyrylcholinesterase activity based on thiamine luminescence modulated by MnO2 nanosheets. Talanta 224:121831. https://doi.org/10.1016/j.talanta.2020.121831
Ma Z, Li P, Jiao M, Shi YE, Zhai Y, Wang Z (2021) Ratiometric sensing of butyrylcholinesterase activity based on the MnO2 nanosheet–modulated fluorescence of sulfur quantum dots and o-phenylenediamine. Microchim Acta 188(9):1–9. https://doi.org/10.1007/s00604-021-04949-0
Ma J, Ma L, Cao L, Miao Y, Dong J, Shi YE, Wang Z (2021) Point-of-care testing of butyrylcholinesterase activity through modulating the photothermal effect of cuprous oxide nanoparticles. Microchim Acta 188(11):1–8. https://doi.org/10.1007/s00604-021-05033-3
Chen M, Zhang J, Chang J, Li H, Zhai Y, Wang Z (2022) Ultrasensitive detection of butyrylcholinesterase activity based on self-polymerization modulated fluorescence of sulfur quantum dots. Spectrochim Acta A Mol Biomol Spectrosc 269:120756. https://doi.org/10.1016/j.saa.2021.120756
Chen P, Zheng C, Chen C, Huang K, Wang X, Hu P, Geng J (2020) Thiol inhibition of Hg cold vapor generation in SnCl2/NaBH4 system: a homogeneous bioassay for H2O2/glucose and butyrylcholinesterase/pesticide sensing by atomic spectrometry. Anal Chim Acta 1111:8–15. https://doi.org/10.1016/j.aca.2020.03.031
Li T, Gao Y, Li H, Zhang C, Xing Y, Jiao M, Shi Y, Li W, Zhai Y, Wang Z (2020). Ultrasensitive detection of butyrylcholinesterase activity based on the inner filter effect of MnO2 nanosheets on sulfur nanodots. Analyst 145(15):5206–5212. https://doi.org/10.1039/D0AN00939C
Chen M, Zhang J, Chang J, Li H, Zhai Y, Wang Z (2022) Ultrasensitive detection of butyrylcholinesterase activity based on self-polymerization modulated fluorescence of sulfur quantum dots. Spectrochim Acta Part A: Mol Biomol Spectrosc 269:120756. https://doi.org/10.1016/j.saa.2021.120756
Sun Y, Tan H, Li Y (2018) A colorimetric assay for acetylcholinesterase activity and inhibitor screening based on the thiocholine–induced inhibition of the oxidative power of MnO2 nanosheets on 3,3′,5, 5′–tetramethylbenzidine. MicrochimicaActa 185(10):1–8. https://doi.org/10.1007/s00604-018-2974-4
Huang L, Li Z, Guo L (2020) Colorimetric assay of acetylcholinesterase inhibitor tacrine based on MoO2 nanoparticles as peroxidase mimetics. Spectrochimica Acta Part A: Mol Biomol Spectrosc 224:117412. https://doi.org/10.1016/j.saa.2019.117412
Wu Y, Jiao L, Luo X, Xu W, Wei X, Wang H, Zhu C (2019) Oxidase-Like Fe-N-C single-atom nanozymes for the detection of acetylcholinesterase activity. Small 15(43):1903108. https://doi.org/10.1002/smll.201903108
Liu Y, Zhou M, Cao W, Wang X, Wang Q, Li S, Wei H (2019) Light-responsive metal–organic framework as an oxidase mimic for cellular glutathione detection. Anal Chem 91(13):8170–8175. https://doi.org/10.1021/acs.analchem.9b00512
Liu SG, Han L, Li N, Fan YZ, Yang YZ, Li NB, Luo HQ (2019) A ratiometric fluorescent strategy for alkaline phosphatase activity assay based on g-C3N4/CoOOH nanohybrid via target-triggered competitive redox reaction. Sens Actuators B: Chem 283:515–523. https://doi.org/10.1016/j.snb.2018.12.052
Funding
This work is supported by the National Natural Science Foundation of China (No. 21775052 and No. 21575048) and the Science and Technology Development project of Jilin province, China (No. 20180414013GH).
All experiments were performed in compliance with the relevant laws and institutional guidelines, and the writing of informed consent for all samples was obtained from human subjects.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, W., Wang, N., Zhou, X. et al. Co, N co-doped porous carbon-based nanozyme as an oxidase mimic for fluorescence and colorimetric biosensing of butyrylcholinesterase activity. Microchim Acta 189, 363 (2022). https://doi.org/10.1007/s00604-022-05446-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05446-8