Abstract
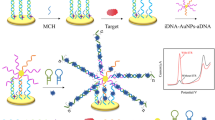
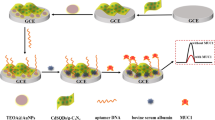
A strand displacement-based “signal-off” electrochemical aptasensor is reported for the detection of Mucin 1 (MUC 1) based on a high original signal. Different from the conventional “signal-off” electrochemical biosensors where electrochemical substances are dispersed in electrolyte solution, here the current signal was generated by the complementary probe (CP) associated with ferrocene (Fc) labeled aptamer (Apt.-Fc). Because Apt.-Fc and MUC 1 have a higher affinity, Apt.-Fc dissociates from CP in the presence of MUC 1, resulting in a reduction of detection current signal generated by oxidation of labeled Fc. In this system, high detection signal is necessary to improve the sensor’s performance. For this aim, a strategy is proposed for changing the modalities of electron transport and the quantity of Apt.-Fc introduced by simply tuning the sequence constitution of CP. As expected, a high detection current signal was obtained after selecting CP(Apt.-Fc)-TTT as the optimal CP. The aptasensor was then employed to detect MUC 1, and satisfactory detection results with a low detection limit (LOD) of 0.087 pM (S/N = 3), good specificity, good stability, and feasibility of detection of MUC 1 in artificial serum (recovery of 92–101%, RSD of 1.36–5.23%) were obtained.
Graphical abstract









Similar content being viewed by others
References
Sha R, Badhulika S (2020) Recent advancements in fabrication of nanomaterial based biosensors for diagnosis of ovarian cancer: a comprehensive review. Microchim Acta 187(3):181. https://doi.org/10.1007/s00604-020-4152-8
Nath S, Mukherjee P (2014) MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med 20(6):332–342. https://doi.org/10.1016/j.molmed.2014.02.007
Li SK, Liu ZT, Li JY, Chen A-Y, Chai Y-Q.; Yuan, R.; Zhuo Y (2018) Enzyme-free target recycling and double-output amplification system for electrochemiluminescent assay of Mucin 1 with MoS2 nanoflowers as co-reaction accelerator. ACS Appl Mater Interfaces 10(17):14483–14490. https://doi.org/10.1021/acsami.8b02262
Yousefi M, Dehghani S, Nosrati R, Zare H, Evazalipour M, Mosafer J, Tehrani BS, Pasdar A, Mokhtarzadeh A, Ramezani M (2019) Aptasensors as a new sensing technology developed for the detection of MUC1 mucin: a review. Biosens Bioelectron 130:1–19. https://doi.org/10.1016/j.bios.2019.01.015
Wang W, Wang Y, Pan H, Cheddah S, Yan C (2019) Aptamer-based fluorometric determination for Mucin 1 using gold nanoparticles and carbon dots. Microc Acta 186(8):544. https://doi.org/10.1007/s00604-019-3516-4
Ferreira CSM, Papamichael K, Guilbault G, Schwarzacher T, Gariepy J, Missailidis S (2008) DNA Aptamers against the MUC1 tumour marker: design of aptamer-antibody sandwich ELISA for the early diagnosis of epithelial tumours. Anal Bioanal Chem 390(4):1039–1050. https://doi.org/10.1007/s00216-007-1470-1
Li N, Zong S, Zhang Y, Wang Z, Wang Y, Zhu K, Yang K, Wang Z, Chen B, Cui Y (2020) A SERS-colorimetric dual-mode aptasensor for the detection of cancer biomarker MUC1. Anal Bioanal Chem 412(23):5707–5718. https://doi.org/10.1007/s00216-020-02790-7
Qin Y, Li D, Yuan R, Xiang Y (2019) Cascaded multiple recycling amplifications for aptamer-based ultrasensitive fluorescence detection of protein biomarkers. Analyst 144(22):6635–6640. https://doi.org/10.1039/c9an01674k
Zhang J, Ran F, Zhou W, Shang B, Yu F, Wu L, Hu W, He X, Chen Q (2018) Ultrasensitive fluorescent aptasensor for MUC1 detection based on deoxyribonuclease I-aided target recycling signal amplification. RSC Advances 8(56):32009–32015. https://doi.org/10.1039/c8ra06498a
Sun J, Li L, Ge S, Zhao P, Zhu P, Wang M, Yu J (2021) Dual-mode aptasensor assembled by a WO3/Fe2O3heterojunction for paper-based colorimetric prediction/photoelectrochemical multicomponent analysis. ACS Appl Mater Interfaces 13(3):3645–3652. https://doi.org/10.1021/acsami.0c19853
Li J, Liu J, Bi Y, Sun M, Bai J, Zhou M (2020) Ultrasensitive electrochemiluminescence biosensing platform for MiRNA-21 and MUC1 detection based on dual catalytic hairpin assembly. Anal Chim Acta 1105:87–94. https://doi.org/10.1016/j.aca.2020.01.034
Sun H, Zu Y (2015) Aptamers and their applications in nanomedicine. Small 11(20):2352–2364. https://doi.org/10.1002/smll.201403073
Ferreira C SM, Matthews CS, Missailidis S (2006) DNA aptamers that bind to MUC1 tumour marker: design and characterization of MUC1-binding single-stranded DNA aptamers. Tumor Biology 27(6):289–301. https://doi.org/10.1159/000096085
Ma F, Ho C, Cheng AKH, Yu HZ (2013) Immobilization of redox-labeled hairpin DNA aptamers on gold: electrochemical quantitation of epithelial tumor marker Mucin 1. Electrochimica Acta 110:139–145. https://doi.org/10.1016/j.electacta.2013.02.088.
Farjami E, Clima L, Gothelf K, Ferapontova EE (2011) “Off-on” electrochemical hairpin-DNA-based genosensor for cancer diagnostics. Anal Chem 83(5):1594–1602. https://doi.org/10.1021/ac1032929
Yang S, Zhang F, Liang Q, Wang Z (2018) Three-dimensional graphene-based ratiometric signal amplification aptasensor for MUC1 detection. Biosens Bioelectron 120:85–92. https://doi.org/10.1016/j.bios.2018.08.036.
Li Y, Liu D, Zhu C, Shen X, Liu Y, You T (2020) Sensitivity programmable ratiometric electrochemical aptasensor based on signal engineering for the detection of aflatoxin B1 in peanut. J Hazard Mater 387:122001. https://doi.org/10.1016/J.JHAZMAT.2019.122001
Zhang Z, Tao C, Yin J, Wang Y, Li Y (2018) Enhancing the response rate of strand displacement-based electrochemical aptamer sensors using bivalent binding aptamer-CDNA probes. Biosens Bioelectron 103:39–44. https://doi.org/10.1016/j.bios.2017.12.027
Pang J, Zhang Z, Jin H (2016) Effect of structure variation of the aptamer-DNA duplex probe on the performance of displacement-based electrochemical aptamer sensors. Biosens Bioelectron 77:174–181. https://doi.org/10.1016/j.bios.2015.09.035
Lou X, Zhao T, Liu R, Ma J, Xiao Y (2013) Self-assembled DNA monolayer buffered dynamic ranges of mercuric electrochemical sensor. Anal Chem 85(15):7574–7580. https://doi.org/10.1021/ac401680c
Deng C, Pi X, Qian P, Chen X, Wu W, Xiang J (2017) High-performance ratiometric electrochemical method based on the combination of signal probe and inner reference probe in one hairpin-structured DNA. Anal Chem 89(1):966–973. https://doi.org/10.1021/acs.analchem.6b04209
Prins R, Korswagen AR, Kortbeek AGTG (1972) Decomposition of the ferricenium cation by nucleophilic reagents. J Organometallic Chem 39(2):335–344. https://doi.org/10.1016/S0022-328X(00)80459-3.
Xiang J, Pi X, Chen X, Xiang, L Yang M, Ren H, Shen X, Qi N, Deng, C (2017) Integrated signal probe based aptasensor for dual-analyte detection. Biosens Bioelectron 96: 268–278. https://doi.org/10.1016/j.bios.2017.04.039
Liu C, Liu X, Qin Y, Deng C, Xiang J (2016) Simple regenerable electrochemical aptasensor for the parallel and continuous detection of biomarkers. RSC Advances 6(63):58469–58476. https://doi.org/10.1039/c6ra09284e
Cheng FF, He TT, Miao HT, Shi JJ, Jiang LP, Zhu JJ, (2015) Electron transfer mediated electrochemical biosensor for microRNAs detection based on metal ion functionalized titanium phosphate nanospheres at attomole level. ACS Appl Mater Interfaces 7(4):2979–2985. https://doi.org/10.1021/am508690x
Wang W, Yuan X, Zhang W, Gao Q, Qi H, Zhang C (2012) Cascade signal amplification for ultra-sensitive impedimetric detection of DNA hybridization using a hairpin DNA as probe. Electrochim Acta 78:377–383. https://doi.org/10.1016/J.ELECTACTA.2012.06.007
Zhu C, Liu D, Li Y, Shen X, Ma S, Liu Y, You T (2020) Ratiometric electrochemical aptasensor for ultrasensitive detection of ochratoxin a based on a dual signal amplification strategy: engineering the binding of methylene blue to DNA. Biosens Bioelectron 150:111814. https://doi.org/10.1016/j.bios.2019.111814
Zhou L, Wang J, Li D, Li Y (2014) An electrochemical aptasensor based on gold nanoparticles dotted graphene modified glassy carbon electrode for label-free detection of bisphenol A in milk samples. Food Chemi 162:34–40. https://doi.org/10.1016/j.foodchem.2014.04.058
Jiang Y, Xiao X, Li C, Luo Y, Chen S, Shi G, Han K, Gu H (2020) Facile ratiometric electrochemical sensor for in vivo/online repetitive measurements of cerebral ascorbic acid in brain microdiaysate. Anal Chem 92(5):3981–3989. https://doi.org/10.1021/acs.analchem.9b05484
Guo J, Wang J, Zhao J, Guo Z, Zhang Y (2016) Ultrasensitive multiplexed immunoassay for tumor biomarkers based on DNA hybridization chain reaction amplifying signal. ACS Appl Mater Interfaces 8(11):6898–6904. https://doi.org/10.1021/acsami.6b00756
Wang L, Ma R, Jiang L, Jia L, Jia W, Wang H (2017) A novel “signal-on/off” sensing platform for selective detection of thrombin based on target-induced ratiometric electrochemical biosensing and bio-bar-coded nanoprobe amplification strategy. Biosens Bioelectron 92:390–395. https://doi.org/10.1016/j.bios.2016.10.089
Slinker JD, Muren NB, Renfrew SE, Barton JK (2011) DNA charge transport over 34 nm. Nature Chem 3(3):228–233. https://doi.org/10.1038/nchem.982
Zhang Z, Dong L, Zhu Q (2018) Rational engineering of synergically stabilized aptamer-CDNA duplex probes for strand displacement based electrochemical sensors. Electrochimica Acta 282:588–594. https://doi.org/10.1016/j.electacta.2018.06.120
Liu R, Yang Z, Guo Q, Zhao J, Ma J, Kang Q, Tang Y, Xue Y, Lou X, He M (2015) Signaling-probe displacement electrochemical aptamer-based sensor (SD-EAB) for detection of nanomolar Kanamycin A. Electrochimica Acta 182:516–523. https://doi.org/10.1016/j.electacta.2015.09.140
Oldham KB (2008) A Gouy-Chapman-Stern model of the double layer at a (metal)/(ionic liquid) interface. J Electroanaytical Chem 613 (2):131–138. https://doi.org/10.1016/j.jelechem.2007.10.017
Deng C, Zhang M, Liu C, Deng H, Huang Y, Yang M, Xiang J, Ren B (2018) Electrostatic force triggering elastic condensation of double-stranded DNA for high-performance one-step immunoassay. Analytical Chem 90 (19):11446–11452. https://doi.org/10.1021/acs.analchem.8b02556
Ma C, Liu H, Zhang L, Li H, Yan M, Song X, Yu J (2018) Multiplexed aptasensor for simultaneous detection of carcinoembryonic antigen and Mucin-1 based on metal ion electrochemical labels and Ru(NH3)63+ electronic wires. Biosens Bioelectron 99:8–13. https://doi.org/10.1016/J.BIOS.2017.07.031
Hong N, Cheng L, Wei BG, Chen CD, He LL, Kong DR, Ceng, JX, Cui HF, Fan H (2017) Fan H An electrochemical DNA sensor without electrode pre-modification. Biosens Bioelectron 91:110–114. https://doi.org/10.1016/j.bios.2016.10.008
Kang D, Zuo X, Yang R, Xia F, Plaxco KW, White RJ (2009) Comparing the properties of electrochemical-based DNA sensors employing different redox tags. Anal Chem 81 (21):9109–9113. https://doi.org/10.1021/ac901811n
Yang W, Lai RYA (2012) Dual-signalling electrochemical DNA sensor based on target hybridization-induced change in DNA probe flexibility. Chem Commun 48 (69):8703–8705. https://doi.org/10.1039/c2cc34312f
Mahato K, Purohit B, Bhardwaj K, Jaiswal A, Chandra P, Novel (2019) Electrochemical biosensor for serotonin detection based on gold nanorattles decorated reduced graphene oxide in biological fluids and in vitro model. Biosens Bioelectron 142:111502. https://doi.org/10.1016/j.bios.2019.111502
Funding
The authors are thankful to the Deanship of Scientific Research at Najran University, Najran, Kingdom of Saudi Arabia, for funding under the Research Collaboration funding program grant no. NU/RC/SERC/11/14. This research was funded by Shandong Provincial Natural Science Foundation (Grant No. ZR2019MB066) and the National Natural Science Foundation of China (Grant No. 21802052).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, C., Guo, W., Umar, A. et al. High-sensitive ferrocene labeled aptasensor for the detection of Mucin 1 by tuning the sequence constitution of complementary probe. Microchim Acta 189, 332 (2022). https://doi.org/10.1007/s00604-022-05424-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05424-0