Abstract
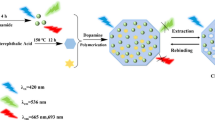
An electromembrane microextraction (EME)-assisted fluorescent molecularly imprinted polymer (MIP) sensing method is presented for detecting the total cathinone drugs in urine samples. In this detection system, the clean-up ability of EME eliminated the matrix effects on both target binding with MIPs and the luminescence of the fluorophore in the sensor. Moreover, by optimizing the extraction conditions of EME, different cathinone drugs with a same concentration show a same response on the single aggregation induced emission (AIE) based MIP (AIE-MIP) sensor (λex = 360 nm, λem = 467 nm). The recoveries were 57.9% for cathinone (CAT) and 78.2% for methcathinone (MCAT). The EME-assisted “light-up” AIE-MIP sensing method displayed excellent performance with a linear range of 2.0–12.0 μmol L−1 and a linear determination coefficient (R2) of 0.99. The limit of detection (LOD) value for EME-assisted “light-up” AIE-MIP sensing method was 0.3 μmol L−1. The relative standard deviation (RSD) values for the detection were found to be within the range 2.0–12.0%. To the best of our knowledge, this is the first time that determination of total illicit drugs with a single fluorescent MIP sensor was achieved and also the first utilization of sample preparation to tune the sensing signal of the sensor to be reported. We believe that this versatile combination of fluorescent MIP sensor and sample preparation can be used as a common protocol for sensing the total amount of a group of analytes in various fields.
Graphical abstract







Similar content being viewed by others
References
Grafinger KE, Liechti ME, Liakoni E (2020) Clinical value of analytical testing in patients presenting with new psychoactive substances intoxication. Br J Clin Pharmacol 86(3):429–436. https://doi.org/10.1111/bcp.14115
Ahmed SR, Chand R, Kumar S, Mittal N, Srinivasan S, Rajabzadeh AR (2020) Recent biosensing advances in the rapid detection of illicit drugs. TrAC Trends Anal Chem 131:116006. https://doi.org/10.1016/j.trac.2020.116006
Xu J, Miao H, Zou L, Bui BTS, Haupt K, Pan G (2021) Evolution of molecularly imprinted enzyme inhibitors: from simple activity inhibition to pathological cell regulation. Angew Chem Int Ed 60:24526–24533. https://doi.org/10.1002/anie.202106657
Ma Y, Yin Y, Ni L, Miao H, Wang Y, Pan C, Tian X, Pan J, You T, Li B, Pan G (2021) Thermo-responsive imprinted hydrogel with switchable sialic acid recognition for selective cancer cell isolation from blood. Bioact Mater 6(5):1308–1317. https://doi.org/10.1016/j.bioactmat.2020.10.008
Li Y, Sun H, Lai J, Chang X, Zhang P, Chen S (2018) Determination of carbonyl pollutants adsorbed on ambient particulate matter of type PM2.5 by using magnetic molecularly imprinted microspheres for sample pretreatment and capillary electrophoresis for separation and quantitation. Microchim Acta 185(2):122–133. https://doi.org/10.1007/s00604-017-2650-0
Tu X, Shi X, Zhao M, Zhang H (2021) Molecularly imprinted dispersive solid-phase microextraction sorbents for direct and selective drug capture from the undiluted bovine serum. Talanta 226:122142. https://doi.org/10.1016/j.talanta.2021.122142
Arabi M, Ostovan A, Li J, Wang X, Zhang Z, Choo J, Chen L (2021) Molecular imprinting: green perspectives and strategies. Adv Mater 33:2100543. https://doi.org/10.1002/adma.202100543
Arabi M, Ostovan A, Zhang Z, Wang Y, Mei R, Fu L, Wang X, Ma J, Chen L (2021) Label-free SERS detection of Raman-inactive protein biomarkers by Raman reporter indicator: Toward ultrasensitivity and universality. Biosens Bioelectron 174:112825. https://doi.org/10.1016/j.bios.2020.112825
Xing R, Guo Z, Lu H, Zhang Q, Liu Z (2021) Molecular imprinting and cladding produces antibody mimics with significantly improved affinity and specificity. Sci Bull 67(3):278–287. https://doi.org/10.1016/j.scib.2021.10.006
Qi J, Li B, Zhou N, Wang X, Deng D, Luo L, Chen L (2019) The strategy of antibody-free biomarker analysis by in-situ synthesized molecularly imprinted polymers on movable valve paper-based device. Biosens Bioelectron 142:111533. https://doi.org/10.1016/j.bios.2019.111533
Chao J, Zeng L, Li R, Zhou Y (2021) Molecularly imprinted polymer-capped wrinkled silica-quantum dot hybrid particles for fluorescent determination of tetra bromo bisphenol A. Microchim Acta 188:126. https://doi.org/10.1007/s00604-021-04779-0
Paredes-Ramos M, Sabin-Lopez A, Pena-Garcia J, Perez-Sanchez H, Lopez-Vilarino JM, Sastre de Vicente ME (2020) Computational aided acetaminophen-phthalic acid molecularly imprinted polymer design for analytical determination of known and new developed recreational drugs. J Mol Graph Model 100:107627. https://doi.org/10.1016/j.jmgm.2020.107627
Mujahid A, Dickert FL (2012) Chapter 6 - Molecularly imprinted polymers for sensors: comparison of optical and mass-sensitive detection. In: Li S, Ge Y, Piletsky SA, Lunec J (eds) molecularly imprinted sensors. Elsevier, Amsterdam, pp 125–159
Yang W, Ma Y, Sun H, Huang C, Shen X (2022) Molecularly imprinted polymers based optical fiber sensors: A review. TrAC Trends Anal Chem 152:116608. https://doi.org/10.1016/j.trac.2022.116608
Chantada-Vázquez MP, Sánchez-González J, Peña-Vázquez E, Tabernero MJ, Bermejo AM, Bermejo-Barrera P, Moreda-Piñeiro A (2016) Synthesis and characterization of novel molecularly imprinted polymer-coated Mn-doped ZnS quantum dots for specific fluorescent recognition of cocaine. Biosens Bioelectron 75:213–221. https://doi.org/10.1016/j.bios.2015.08.022
Masteri-Farahani M, Mashhadi-Ramezani S, Mosleh N (2020) Molecularly imprinted polymer containing fluorescent graphene quantum dots as a new fluorescent nanosensor for detection of methamphetamine. Spectrochim Acta Part A: Mol Biomol Spectrosc 229:118021. https://doi.org/10.1016/j.saa.2019.118021
Merone GM, Tartaglia A, Rossi S, Santavenere F, Bassotti E, D’Ovidio C, Rosato E, de Grazia U, Locatelli M, Boccio PD, Savini F (2022) Fast LC–MS/MS screening method for the evaluation of drugs, illicit drugs, and other compounds in biological matrices. Talanta Open 5:100105. https://doi.org/10.1016/j.talo.2022.100105
Tang MHY, Tong HF, Wong KC, Chong YK (2022) Ropinirole metabolite mimics a new psychoactive substance (4-HO-MET) in LC-MS/MS. Forensic Sci Int 331:111151. https://doi.org/10.1016/j.forsciint.2021.111151
Anzar N, Suleman S, Parvez S, Narang J (2022) A review on illicit drugs and biosensing advances for its rapid detection. Process Biochem 113:113–124. https://doi.org/10.1016/j.procbio.2021.12.021
Zeng L, Zhang X, Wang X, Cheng D, Li R, Han B, Wu M, Zhuang Z, Ren A, Zhou Y, Jing T (2021) Simultaneous fluorescence determination of bisphenol A and its halogenated analogs based on a molecularly imprinted paper-based analytical device and a segment detection strategy. Biosens Bioelectron 180:113106. https://doi.org/10.1016/j.bios.2021.113106
Duan YP, Dai CM, Zhang YL, Chen L (2013) Selective trace enrichment of acidic pharmaceuticals in real water and sediment samples based on solid-phase extraction using multi-templates molecularly imprinted polymers. Anal Chim Acta 758:93–100. https://doi.org/10.1016/j.aca.2012.11.010
Ostovan A, Ghaedi M, Arabi M, Yang Q, Li J, Chen L (2018) Hydrophilic multitemplate molecularly imprinted biopolymers based on a green synthesis strategy for determination of B-family vitamins. ACS Appl Mater Interfaces 10:4140–4150. https://doi.org/10.1021/acsami.7b17500
Zhang M, He J, Shen Y, He W, Li Y, Zhao D, Zhang S (2018) Application of pseudo-template molecularly imprinted polymers by atom transfer radical polymerization to the solid-phase extraction of pyrethroids. Talanta 178:1011–1016. https://doi.org/10.1016/j.talanta.2017.08.100
Arabi M, Ostovan A, Bagheri AR, Guo X, Wang L, Li J, Wang X, Li B, Chen L (2020) Strategies of molecular imprinting-based solid-phase extraction prior to chromatographic analysis. Trends Anal Chem 128:115923. https://doi.org/10.1016/j.trac.2020.115923
Mujahid A, Afzal A, Glanzing G, Leidl A, Lieberzeit PA, Dickert FL (2010) Imprinted sol–gel materials for monitoring degradation products in automotive oils by shear transverse wave. Anal Chim Acta 675(1):53–57. https://doi.org/10.1016/j.aca.2010.07.005
Zhang XM, Qin YP, Ye HL, Ma XT, He XW, Li WY, Zhang YK (2018) Silicon nanoparticles coated with an epitope-imprinted polymer for fluorometric determination of cytochrome c. Microchim Acta 185:173. https://doi.org/10.1007/s00604-018-2724-7
Xu C, Uddin KMA, Shen X, Jayawardena HSN, Yan M, Ye L (2013) Photoconjugation of molecularly imprinted polymer with magnetic nanoparticles. ACS Appl Mater Interfaces 5(11):5208–5213. https://doi.org/10.1021/am401042u
Haupt K, Dzgoev A, Mosbach K (1998) Assay system for the herbicide 2,4-dichlorophenoxyacetic acid using a molecularly imprinted polymer as an artificial recognition element. Anal Chem 70(3):628–631. https://doi.org/10.1021/ac9711549
Lieberzeit PA, Afzal A, Glanzing G, Dickert FL (2007) Molecularly imprinted sol–gel nanoparticles for mass-sensitive engine oil degradation sensing. Anal Bioanal Chem 389:441–446. https://doi.org/10.1007/s00216-007-1274-3
Yan Y, Jiang L, Zhang S, Shen X, Huang C (2022) Specific “light-up” sensor made easy: an aggregation induced emission monomer for molecular imprinting. Biosens Bioelectron 205:114113. https://doi.org/10.1016/j.bios.2022.114113
Teng Y, Liu F, Kan X (2017) Voltammetric dopamine sensor based on three-dimensional electrosynthesized molecularly imprinted polymers and polypyrrole nanowires. Microchim Acta 184:2515–2522. https://doi.org/10.1007/s00604-017-2243-y
González-Mariño I, Gracia-Lor E, Rousis NI, Castrignanò E, Thomas KV, Quintana JB, Kasprzyk-Hordern B, Zuccato E, Castiglioni S (2016) Wastewater-based epidemiology to monitor synthetic cathinones use in different European countries. Environ Sci Technol 50(18):10089–10096. https://doi.org/10.1021/acs.est.6b02644
O’Rourke CE, Subedi B (2020) Occurrence and mass loading of synthetic opioids, synthetic cathinones, and synthetic cannabinoids in wastewater treatment plants in four U.S. communities. Environ Sci Technol 54(11):6661–6670. https://doi.org/10.1021/acs.est.0c00250
Huang C, Shen X, Gjelstad A, Pedersen-Bjergaard S (2018) Investigation of alternative supported liquid membranes in electromembrane extraction of basic drugs from human plasma. J Membrane Sci 548:176–183. https://doi.org/10.1016/j.memsci.2018.09.056
Capoferri D, Carlo MD, Ntshongontshi N, Iwuoha EI, Sergi M, Ottavio FD, Compagnone D (2017) MIP-MEPS based sensing strategy for the selective assay of dimethoate. Application to wheat flour samples. Talanta 174:599–604. https://doi.org/10.1016/j.talanta.2017.06.062
Pedersen-Bjergaard S, Rasmussen KE (2006) Electrokinetic migration across artificial liquid membranes: new concept for rapid sample preparation of biological fluids. J Chromatogr A 1109(2):183–190. https://doi.org/10.1016/j.chroma.2006.01.025
Drouin N, Kubáň P, Rudaz S, Pedersen-Bjergaard S, Schappler J (2019) Electromembrane extraction: overview of the last decade. TrAC Trends Anal Chem 113:357–363. https://doi.org/10.1016/j.trac.2018.10.024
Seip KF, Gjelstad A, Pedersen-Bjergaard S (2013) Electromembrane extraction from aqueous samples containing polar organic solvents. J Chromatogr A 1308:37–44. https://doi.org/10.1016/j.chroma.2013.07.105
Hong C, Dong Y, Zhu R, Yan Y, Shen X, Pedersen-Bjergaard S, Huang C (2022) Effect of sample matrices on supported liquid membrane: efficient electromembrane extraction of cathinones from biological samples. Talanta 240:123175. https://doi.org/10.1016/j.talanta.2021.123175
Aldubayyan AA, Castrignanò E, Elliott S, Abbate V (2021) Stability of synthetic cathinones in clinical and forensic toxicological analysis—where are we now? Drug Test Anal 13:44–68. https://doi.org/10.1002/dta.2990
Bijlsma L, Celma A, Castiglioni S, Salgueiro-González N, Bou-Iserte L, Baz-Lomba JA, Reid MJ, Dias MJ, Lopes A, Matias J, Pastor-Alcañiz L, Radonić J, Turk Sekulic M, Shine T, van Nuijs ALN, Hernandez F, Zuccato E (2020) Monitoring psychoactive substance use at six European festivals through wastewater and pooled urine analysis. Sci Total Environ 725:138376. https://doi.org/10.1016/j.scitotenv.2020.138376
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant numbers 81801875 and 21874050).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, R., Yan, Y., Jiang, L. et al. Determination of total cathinones with a single molecularly imprinted fluorescent sensor assisted by electromembrane microextraction. Microchim Acta 189, 324 (2022). https://doi.org/10.1007/s00604-022-05405-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05405-3