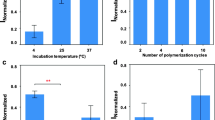
Abstract
In contrast to reported enzyme-based immunoassays, an enzyme-free immunoassay (optical and electrochemical) is presented here for the first time that can be used as point-of-need detection bioplatforms of bovine IgG as goat milk adulterant. In the first format, Prussian blue nanoparticles (PBNPs) were used as antibody catalytic labels in a competitive colorimetric microplate immunoassay. Absorbance measurement was performed photometrically at 450 nm. After in-depth optimization, excellent sensitivity was achieved (0.01% cow/goat volume ratio), which is 100 times lower than the limit allowed by the European legislation (EL) (1% v/v), thanks to the high catalytic activity of PBNPs compared with natural peroxidase. Moreover, the antibody-PBNPs bioconjugates showed excellent stability over 4 weeks (> 94% of the initial response) confirming the successful anchoring of the antibodies to the surface of the PBNPs. On the other hand, a label-free voltammetric immunoassay for the detection of bovine IgG was developed. The sensing principle was based on the hindrance of charge transfer between ferri-ferrocyanide redox couple and the screen-printed gold electrodes modified with bovine IgG antibody. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were used to characterize the step-by-step modification of the electrode surface. Under optimal conditions, this single-step electrochemical analysis achieved a high sensitivity of 0.1% (cow/goat) when monitoring the ferrocyanide oxidation at + 0.092 V (vs. Ag/AgCl) using differential pulse voltammetry (DPV). The selectivity of the developed immunoassays was evaluated for different species of milk of similar composition, and both immunoassays exhibited a selective response only to bovine IgG. Unlike conventional immunoassays, the developed enzyme-free immunoassays have many attractive features for the detection of milk adulteration, whether they are used in quality control laboratories for routine milk analysis (optical immunoassay) or at on-site checkpoints (electrochemical immunoassay) using wireless electrochemical detectors. The sensors provide high sensitivity (≤ 0.1%), excellent precision (RSD < 6%), low cost (no enzyme is required) and ease of operation, including handling of milk samples.
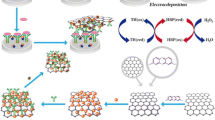
Graphical abstract







Similar content being viewed by others
References
Selamat J, Iqbal SZ (2016) Food safety: Basic concepts, recent issues, and future challenges Food Saf Basic Concepts Recent Issues Futur Challenges 1–160. https://doi.org/10.1007/978-3-319-39253-0
Mu WY, Huang PZ, Chen QY, Wang W (2020) Determination of melamine and melamine–Cu(II) complexes in milk using a DNA-Ag hydrocolloid as the sensor. Food Chem 311:125889. https://doi.org/10.1016/j.foodchem.2019.125889
Deng L, Li A, Gao Y, Shen T, Yue H, Miao J, Li R, Yang J (2020) Detection of the bovine milk adulterated in camel, horse, and goat milk using Duplex PCR. Food Anal Methods 13:560–567. https://doi.org/10.1007/s12161-019-01678-2
Poonia A, Jha A, Sharma R, Singh HB, Rai AK, Sharma N (2017) Detection of adulteration in milk: A review. Int J Dairy Technol 70:23–42. https://doi.org/10.1111/1471-0307.12274
Ozer D, Sezer B, Durna S, Hakki I (2021) Determination of milk fat authenticity in ultra- fi ltered white cheese by using Raman spectroscopy with multivariate data analysis. Food Chem 336:127699. https://doi.org/10.1016/j.foodchem.2020.127699
Singh N (2019) Ashish Kumar, SINGH, Minni, et VERMA, Electrochemical preparation of Fe3O4/MWCNT-polyaniline nanocomposite film for development of urea biosensor and its application in milk sample. J Food Meas Charact 14:163–175. https://doi.org/10.1007/s11694-019-00278-2
Regasa MB, Soreta TR, Femi OE, Ramamurthy PC (2020) Development of molecularly imprinted conducting polymer composite film-based electrochemical sensor for melamine detection in infant formula. ACS Omega 5:4090–4099. https://doi.org/10.1021/acsomega.9b03747
Lin C, Zhong C, Song Y, Wang L (2021) Ratiometric fluorescence detection of melamine in milk by a zirconium-based metal-organic frameworks composite. Microchem J 162:105837. https://doi.org/10.1016/j.microc.2020.105837
Nagraik R, Sharma A, Kumar D, Chawla P, Kumar AP (2021) Milk adulterant detection: Conventional and biosensor based approaches: A review. Sens Bio-Sensing Res 33:100433. https://doi.org/10.1016/j.sbsr.2021.100433
Li L, Wang J, Li M, Yang Y, Wang Z, Miao J, Zhao Z, Yang J (2021) Detection of the adulteration of camel milk powder with cow milk by ultra-high performance liquid chromatography (UPLC). Int Dairy J 121:105117. https://doi.org/10.1016/j.idairyj.2021.105117
Sousa YRF, Medeiros LB, Pintado MME, Queiroga RCRE (2019) Goat milk oligosaccharides: Composition, analytical methods and bioactive and nutritional properties. Trends Food Sci Technol 92:152–161. https://doi.org/10.1016/j.tifs.2019.07.052
dos Santos Pereira, Elainy Virginia, de Sousa Fernandes DD, Araújo MCU, Diniz PHGD, Maciel MIS (2020) Simultaneous determination of goat milk adulteration with cow milk and their fat and protein contents using NIR spectroscopy and PLS algorithms. LWT 127:109427. https://doi.org/10.1016/j.lwt.2020.109427
El Hawiet A, Simple A (2018) Sensitive, and label - free platform for the quantification of lactoferrin in camel and goat milk based on thin - layer chromatography. Chromatographia 80:1797–1804. https://doi.org/10.1007/s10337-017-3414-z
Trimboli F, Morittu VM, Cicino C, Palmieri C, Britti D (2017) Rapid capillary electrophoresis approach for the quantification of ewe milk adulteration with cow milk. J Chromatogr A 1519:131–136. https://doi.org/10.1016/j.chroma.2017.08.075
Genis DO, Sezer B, Bilge G, Durna S, Boyaci IH (2020) Development of synchronous fluorescence method for identification of cow, goat, ewe and buffalo milk species. Food Control 108:106808. https://doi.org/10.1016/j.foodcont.2019.106808
Di Pinto A, Terio V, Marchetti P, Bottaro M, Mottola A, Bozzo G, Bonerba E, Ceci E, Tantillo G (2017) DNA-based approach for species identification of goat-milk products. Food Chem 229:93–97. https://doi.org/10.1016/j.foodchem.2017.02.067
Hurley IP, Coleman RC, Ireland HE, Williams JHH (2006) Use of sandwich IgG ELISA for the detection and quantification of adulteration of milk and soft cheese. Int Dairy J 16:805–812. https://doi.org/10.1016/j.idairyj.2005.07.009
Ren QR, Zhang H, Guo HY, Jiang L, Tian M, Ren FZ (2014) Detection of cow milk adulteration in yak milk by ELISA. J Dairy Sci 97:6000–6006. https://doi.org/10.3168/jds.2014-8127
Song H, Xue H, Han Y (2011) Detection of cow’s milk in Shaanxi goat’s milk with an ELISA assay. Food Control 22:883–887. https://doi.org/10.1016/j.foodcont.2010.11.019
Sharma R, Verma A, Shinde N, Mann B, Gandhi K, Wichers JH, van Amerongen A (2021) Adulteration of cow’s milk with buffalo’s milk detected by an on-site carbon nanoparticles-based lateral flow immunoassay. Food Chem 351:129311. https://doi.org/10.1016/j.foodchem.2021.129311
Liu B, Si J, Zhao F, Wang Q, Wang Y, Li J, Li C, Li T (2019) Rapid detection of cow milk adulteration/contamination in goat milk by a lateral flow colloidal gold immunoassay strip. J Dairy Res 86:94–97. https://doi.org/10.1017/S0022029919000116
Ruiz-Valdepeñas Montiel V, Povedano E, Benedé S, Mata L, Galán-Malo P, Gamella M, Reviejo AJ, Campuzano S, Pingarrón JM (2019) Disposable Amperometric Immunosensor for the detection of adulteration in milk through single or multiplexed determination of Bovine, Ovine, or Caprine Immunoglobulins G. Anal Chem 91:11266–11274. https://doi.org/10.1021/acs.analchem.9b02336
Zhang R, Yan X, Fan K (2021) Nanozymes inspired by natural enzymes, accounts. Mater Res 2:534–547. https://doi.org/10.1021/accountsmr.1c00074
Zhang R, Fan K, Yan X (2020) Nanozymes: created by learning from nature. Sci China Life Sci 63:1183–1200. https://doi.org/10.1007/s11427-019-1570-7
Niu X, Cheng N, Ruan X, Du D, Lin Y (2020) Review — Nanozyme-Based Immunosensors and Immunoassays : recent developments and future trends. J Electrochem Soc 167:037508. https://doi.org/10.1149/2.0082003JES
Huang P, Yan X, Cai W (2019) Nanozyme: new horizons for responsive biomedical applications. Chem Soc Rev 48:3683–3704. https://doi.org/10.1039/c8cs00718g
Ricci F, Amine A, Palleschi G, Moscone D (2003) Prussian Blue based screen printed biosensors with impro v ed characteristics of long-term lifetime and pH stability. Biosens Bioelectron 18:165–174
Wang Q, Wei H, Zhang Z, Wang E, Dong S (2018) Nanozyme: An emerging alternative to natural enzyme for biosensing and immunoassay, TrAC -. Trends Anal Chem 105:218–224. https://doi.org/10.1016/j.trac.2018.05.012
Singh S (2019) Nanomaterials exhibiting enzyme-like properties ( Nanozymes ): current advances and future perspectives. Front Chem 7:1–10. https://doi.org/10.3389/fchem.2019.00046
Estelrich J, Ant M (2021) Prussian blue : A nanozyme with versatile catalytic properties. Int J Mole 22:5993. https://doi.org/10.3390/ijms22115993
Komkova MA, Karyakina EE, Karyakin AA, Komkova MA, Karyakina EE, Karyakin AA (2018) Catalytically synthesized Prussian Blue nanoparticles defeating natural enzyme peroxidase Catalytically synthesized Prussian Blue nanoparticles defeat- ing natural enzyme peroxidase. J Am Chem Soc 140:11302–11307. https://doi.org/10.1021/jacs.8b05223
Komkova MA, Ibragimova OA, Karyakina EE, Karyakin AA (2021) Catalytic pathway of nanozyme “ Artificial Peroxidase ” with 100-Fold greater bimolecular rate constants compared to those of the enzyme. J Phys Chem Lett 12:171–176. https://doi.org/10.1021/acs.jpclett.0c03014
Attar A, Mandli J, Ennaji M, Amine A (2016) Label-free electrochemical impedance detection of rotavirus based on immobilized antibodies on gold sononanoparticles. Electroanalysis 28:1839–1846. https://doi.org/10.1002/elan.201600179
Farka Z, Čunderlová V, Horáčková V, Pastucha M, Mikušová Z, Hlaváček A, Skládal P (2018) Prussian blue nanoparticles as a catalytic label in a sandwich nanozyme-linked immunosorbent assay. Anal Chem 90:2348–2354. https://doi.org/10.1021/acs.analchem.7b04883
Seddaoui N, Amine A (2021) Smartphone-based competitive immunoassay for quantitative on-site detection of meat adulteration. Talanta 230:122346. https://doi.org/10.1016/j.talanta.2021.122346
Farrell HM, Jimenez-Flores R, Bleck GT, Brown EM, Butler JE, Creamer LK, Hicks CL, Hollar CM, Ng-Kwai-Hang KF, Swaisgood HE (2004) Nomenclature of the proteins of cows’ milk - Sixth revision. J Dairy Sci 87:1641–1674. https://doi.org/10.3168/jds.S0022-0302(04)73319-6
Ke X, Zhang J, Lai S, Chen Q, Zhang Y, Jiang Y, Mo W, Ren Y (2017) Quantitative analysis of cow whole milk and whey powder adulteration percentage in goat and sheep milk products by isotopic dilution-ultra-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 409:213–224. https://doi.org/10.1007/s00216-016-9987-9
Dvorak L, Mlcek J, Sustova K (2016) Comparison of FT-NIR spectroscopy and ELISA for detection of adulteration of goat cheeses with cow’s milk. J AOAC Int 99:180–186. https://doi.org/10.5740/jaoacint.15-0190
Yaman H (2020) A rapid method for detection adulteration in goat milk by using vibrational spectroscopy in combination with chemometric methods. J Food Sci Technol 57:3091–3098. https://doi.org/10.1007/s13197-020-04342-4
Hurley IP, Coleman RC, Ireland HE, Williams JHH (2004) Measurement of bovine IgG by indirect competitive ELISA as a means of detecting milk adulteration. J Dairy Sci 87:543–549. https://doi.org/10.3168/jds.S0022-0302(04)73195-1
Galan-Malo P, Mendiara I, Razquin P, Mata L (2018) Validation of a rapid lateral flow method for the detection of cows’ milk in water buffalo, sheep or goat milk. Food Addit Contam - Part A Chem Anal Control Expo Risk Assess 35:599–604. https://doi.org/10.1080/19440049.2018.1426886
Stǎnciuc Sava N, Râpeanu G (2010) Identification of adulterated sheep and goat cheeses marketed in Romania by immunocromatographic assay. Food Agric Immunol 21:157–164. https://doi.org/10.1080/09540100903508683
Seddaoui N, Amine A (2020) A sensitive colorimetric immunoassay based on poly (dopamine) modified magnetic nanoparticles for meat authentication. LWT - Food Sci Technol 122:109045. https://doi.org/10.1016/j.lwt.2020.109045
Sarode AR, Sawale PD, Khedkar CD, Kalyankar SD, Pawshe RD (2015) Casein and caseinate: methods of manufacture, 1st edn. Elsevier Ltd., Amsterdam. https://doi.org/10.1016/B978-0-12-384947-2.00122-7
Gautam M, Poudel K, Yong CS, Kim JO (2018) Prussian blue nanoparticles: Synthesis, surface modification, and application in cancer treatment. Int J Pharm 549:31–49. https://doi.org/10.1016/j.ijpharm.2018.07.055
Gu S, Risse S, Lu Y, Ballauff M (2020) Mechanism of the oxidation of 3,3′,5,5′-Tetramethylbenzidine catalyzed by Peroxidase-Like Pt nanoparticles immobilized in spherical polyelectrolyte brushes: A Kinetic study. ChemPhysChem 21:450–458. https://doi.org/10.1002/cphc.201901087
Hermanson GT (2013) Bioconjugate techniques. Academic press
Narain R (Ed.) (2013) Chemistry of bioconjugates: synthesis, characterization, and biomedical applications. John Wiley & Sons
Vashist SK (2012) Comparison of 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide based strategies to crosslink antibodies on amine-functionalized platforms for immunodiagnostic applications. Diagnostics 2:23–33. https://doi.org/10.3390/diagnostics2030023
Moore CJ, Montón H, O’Kennedy R, Williams DE, Nogués C, Crean C, Gubala V (2015) Controlling colloidal stability of silica nanoparticles during bioconjugation reactions with proteins and improving their longer-term stability, handling and storage. J Mater Chem B 3:2043–2055. https://doi.org/10.1039/c4tb01915f
Parliament E (2008) Commission regulation (EC) No 273/2008. Off J Eur Union 43:1–5
Beer M, Krause I, Stapf M, Schwarzer C, Klostermeyer H (1996) Indirect competitive enzyme-linked immunosorbent assay for the detection of native and heat-denatured bovine β-lactoglobulin in ewes’ and goats’ milk cheese. Eur Food Res Technol 203:21–26. https://doi.org/10.1007/BF01267764
Liv L (2021) Electrochemical immunosensor platform based on gold-clusters, cysteamine and glutaraldehyde modified electrode for diagnosing COVID-19. Microchem J 168:106445. https://doi.org/10.1016/j.microc.2021.106445
Author information
Authors and Affiliations
Contributions
Narjiss Seddaoui contributed to conceptualization, methodology, validation, investigation, writing—original draft preparation, visualization and writing—review and editing. Raouia Attaallah contributed to conceptualization, investigation and writing—review and editing. Aziz Amine contributed to conceptualization, methodology, validation, writing—review and editing, and supervision.
Corresponding author
Ethics declarations
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Seddaoui, N., Attaallah, R. & Amine, A. Development of an optical immunoassay based on peroxidase-mimicking Prussian blue nanoparticles and a label-free electrochemical immunosensor for accurate and sensitive quantification of milk species adulteration. Microchim Acta 189, 209 (2022). https://doi.org/10.1007/s00604-022-05302-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05302-9