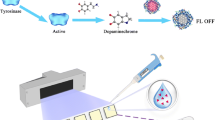
Abstract
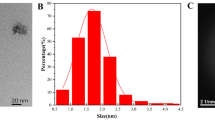
A rapid and convenient fluorescence glyphosate (GLYP) biosensor was developed based on DNA-templated copper nanoparticles (DNA-CuNPs). In the absence of GLYP, the DNA-CuNPs were formed through the reduction of Cu2+ by vitamin C (Vc). The DNA-CuNPs emitted intense fluorescence at 615 nm when being excited at 340 nm. In the presence of GLYP, GLYP can strongly chelate with Cu2+ by the phosphate and carboxyl groups to decrease the amount of free Cu2+. Due to the lack of free Cu2+, DNA-CuNPs cannot be formed, which caused the fluorescence to decrease. The whole detection process of this proposed GLYP biosensor can be completed within 14 min. Titration experiments showed that this biosensor had a linear relationship for GLYP in the range 1 to 18 µM with a limit of detection (LOD) of 0.47 µM. This biosensor showed obvious selectivity among other pesticides, even between GLYP and organophosphorus pesticides. This biosensor performed well for GLYP detection in real samples with recoveries of 88.0–104.0%.
Graphical abstract





Similar content being viewed by others
References
Soukup ST, Merz B, Bub A, Hoffmann I, Watzl B, Steinberg P, Kulling SE (2020) Glyphosate and AMPA levels in human urine samples and their correlation with food consumption: results of the cross-sectional KarMeN study in Germany. Arch Toxicol 94:1575–1584
Parlapiano I, Biandolino F, Grattagliano A, Ruscito A, Libralato G, Prato E (2021) Effects of commercial formulations of glyphosate on marine crustaceans and implications for risk assessment under temperature changes. Ecotoxicol Environ Saf 213:112068
Tsai WT (2019) Trends in the use of glyphosate herbicide and its relevant regulations in Taiwan: a water contaminant of increasing concern. Toxics 7:4
Bailey DC, Todt CE, Burchfield SL, Pressley AS, Denney RD, Snapp IB, Negga R, Traynor WL (2018) Chronic exposure to a glyphosate-containing pesticide leads to mitochondrial dysfunction and increased reactive oxygen species production in Caenorhabditis elegans. Environ Toxicol Phar 57:46–52
Gunatilake S, Seneff S, Orlando L (2019) Glyphosate’s synergistic toxicity in combination with other factors as a cause of chronic kidney disease of unknown origin. Int J Environ Res Public Health 16:2734
Mills PJ, Caussy C, Loomba R (2020) Glyphosate excretion is associated with steatohepatitis and advanced liver fibrosis in patients with fatty liver disease. Clin Gastroenterol Hepatol 18:741–743
Guan JP, Yang J, Zhang Y, Zhang XX, Deng HJ, Xu J, Wang JY, Yuan MS (2021) Employing a fluorescent and colorimetric picolyl-functionalized rhodamine for the detection of glyphosate pesticide. Talanta 224:121834
Valle AL, Mello F, Alves-Balvedi RP, Rodrigues LP, Goulart LR (2018) Glyphosate detection: methods, needs and challenges. Environ Chem Lett 17:291–317
Sun L, Kong D, Gu W, Guo X, Tao W, Shan Z, Wang Y, Wang N (2017) Determination of glyphosate in soil/sludge by high performance liquid chromatography. J chromatogr A 1502:8–13
Zhang W, Feng Y, Ma L, An J, Zhang H, Cao M, Zhu H, Kang W, Lian K (2019) A method for determining glyphosate and its metabolite aminomethyl phosphonic acid by gas chromatography-flame photometric detection. J Chromatogr A 1589:116–121
Viirlaid E, Ilisson M, Kopanchuk S, Mäeorg U, Rinken A, Rinken T (2019) Immunoassay for rapid on-site detection of glyphosate herbicide. Environ Monit Assess 191:507
Ibarra Bouzada LME, Hernández SR, Kergaravat SV (2019) Glyphosate detection from commercial formulations: comparison of screening analytic methods based on enzymatic inhibition. Int J Environ Anal Chem 101:1821–1835
Bettazzi F, Romero Natale A, Torres E, Palchetti I (2018) Glyphosate determination by coupling an immuno-magnetic assay with electrochemical sensors. Sensors-Basel 18:2965
Tan C, Liu G, Li H, Cui Y, Liu Y (2020) Ultrathin two-dimensional metal-organic framework nanosheets-an emerging class of catalytic nanomaterials. Dalton Trans 49:11073–11084
Hou J, Wang X, Lan S, Zhang C, Hou C, He Q, Huo D (2020) A turn-on fluorescent sensor based on carbon dots from Sophora japonica leaves for the detection of glyphosate. Anal Methods 12:4130–4138
Gui M, Jiang J, Wang X, Yan Y, Li S, Xiao X, Liu T, Liu T, Feng Y (2017) Copper ion-mediated glyphosate detection with N-heterocycle based polyacetylene as a sensing platform. Sensor Actuat B-Chem 243:696–703
Qing Z, Bai A, Xing S, Zou Z, He X, Wang K, Yang R (2019) Progress in biosensor based on DNA-templated copper nanoparticles. Biosen Bioelectron 137:96–109
Rotaru A, Dutta S, Jentzsch E, Gothelf K, Mokhir A (2010) Selective dsDNA-templated formation of copper nanoparticles in solution. Angew Chem Int Ed Engl 49:5665–5667
Qing Z, He X, He D, Wang K, Xu F, Qing T, Yang X (2013) Poly(thymine)-templated selective formation of fluorescent copper nanoparticles. Ang Chem 52:9719–9722
Qing TP, Qing ZH, Mao ZG, He XX, Xu FZ, Wen L, He DG, Shi H, Wang KM (2014) dsDNA-templated fluorescent copper nanoparticles: poly(AT-TA)-dependent formation. Rsc Adv 4:61092–61095
Song Q, Shi Y, He D, Xu S, Ouyang J (2015) Sequence-dependent dsDNA-templated formation of fluorescent copper nanoparticles. Chem Eur J 21:2417–2422
Qing T, Zhang K, Qing Z, Wang X, Long C, Zhang P, Hu H, Feng B (2019) Recent progress in copper nanocluster-based fluorescent probing: a review. Microchim Acta 186:670
Qing Z, Mao Z, Qing T, He X, Zou Z, He D, Shi H, Huang J, Liu J, Wang K (2014) Visual and portable strategy for copper(II) detection based on a striplike poly(thymine)-caged and microwell-printed hydrogel. Anal Chem 86:11263–11268
Zhu Y, Liu X, Liu K, Bao X, Cheng S, Zhang L, Zhang Y, Zhang L, Cao F, Xing X (2020) Enhanced-assay of alkaline phosphatase based on polyAT dsDNA-templated copper nanoclusters. Microchem J 158:105165
Song Q, Wang R, Sun F, Chen H, Wang Z, Na N, Ouyang J (2017) A nuclease-assisted label-free aptasensor for fluorescence turn-on detection of ATP based on the in situ formation of copper nanoparticles. Biosensor Bioelectron 87:760–763
Qu F, Wang H, You JM (2020) Dual lanthanide-probe based on coordination polymer networks for ratiometric detection of glyphosate in food samples. Food Chem 323:126815
Sheals J, Persson P, Hedman B (2001) IR and EXAFS spectroscopic studies of glyphosate protonation and copper(II) complexes of glyphosate in aqueous solution. Inorg Chem 40:4302–4309
Wang X, Yang Y, Huo D, Ji Z, Ma Y, Yang M, Luo H, Luo X, Hou C, Lv J (2020) A turn-on fluorescent nanoprobe based on N-doped silicon quantum dots for rapid determination of glyphosate. Microchim Acta 187:341
Liu Z, Yang L, Sharma AS, Chen M, Chen Q (2019) A system composed of polyethylenimine-capped upconversion nanoparticles, copper(II), hydrogen peroxide and 3,3′,5,5′-tetramethylbenzidine for colorimetric and fluorometric determination of glyphosate. Microchim Acta 186:835
Jain S, Jain A, Kachhawah P, Devra V (2015) Synthesis and size control of copper nanoparticles and their catalytic application. T Nonferr Metal Soc 25:3995–4000
Xiong Y, Gao B, Wu KS, Wu YQ, Chai YJ, Huang XL, Xiong YH (2018) Fluorescence immunoassay based on the enzyme cleaving ss-DNA to regulate the synthesis of histone-ds-poly(AT) templated copper nanoparticles. Nanoscale 10:19890–19897
Chen Q, Chen H, Li Z, Pang J, Lin T, Guo L, Fu F (2017) Colorimetric sensing of glyphosate in environmental water based on peroxidase mimetic activity of MoS2 nanosheets. J Nanosci Nanotechnol 17:5730–5734
Yuan Y, Jiang J, Liu S, Yang J, Zhang H, Yan J, Hu X (2017) Fluorescent carbon dots for glyphosate determination based on fluorescence resonance energy transfer and logic gate operation. Sensor Actua B-Chem 242:545–553
Ma J, Feng G, Ying Y, Shao Y, She Y, Zheng L, Abd Ei-Aty AM, Wang J (2021) Sensitive SERS assay for glyphosate based on the prevention of l-cysteine inhibition of a Au-Pt nanozyme. Analyst 146:956–963
Wang L, Bi Y, Gao J, Li Y, Ding H, Ding L (2016) Carbon dots based turn-on fluorescent probes for the sensitive determination of glyphosate in environmental water samples. Rsc Adv 6:85820–85828
Chang Y, Zhang Z, Hao J, Yang W, Tang J (2016) A simple label free colorimetric method for glyphosate detection based on the inhibition of peroxidase-like activity of Cu(II). Sensor Actuat B-Chem 228:410–415
Fang F, Wei R, Liu X (2014) Novel pre-column derivatisation reagent for glyphosate by high-performance liquid chromatography and ultraviolet detection. Int J Environ Anal Chem 94:661–667
Madsen HEL, Christensen HH, Petersen CG (1978) Stability constants of copper(II), zinc, manganese(II), calcium, and magnesium complexes of N-(phosphonomethyl)glycine (glyphosate). Acta Chem Scand A 32:79–83
Zhang P, Wang Y, Chang Y, Xiong ZH, Huang CZ (2013) Highly selective detection of bacterial alarmone ppGpp with an off-on fluorescent probe of copper-mediated silver nanoclusters. Biosens Bioelectron 49:433–437
Funding
The work is supported by the National Natural Science Foundation of China (31601536, 31871888), and State Environmental Protection Key Laboratory of Synergetic Control and Joint Remediation for Soil & Water Pollution (GHBK-2020–001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fang, H., Zhang, X., Gao, D. et al. Fluorescence determination of glyphosate based on a DNA-templated copper nanoparticle biosensor. Microchim Acta 189, 158 (2022). https://doi.org/10.1007/s00604-022-05284-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05284-8