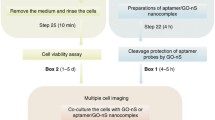
Abstract
The development of an intracellular metabolite imaging platform for live microorganisms has been a challenge in the study of microbes. Herein, we performed metabolite imaging in live microalgal cells using a graphene oxide (GO)/aptamer complex. The properties of the GO were characterized using dynamic light scattering (DLS) and atomic force microscopy (AFM), which were determined to have 140 ± 3 nm in mean diameter. An ATP-specific aptamer was mixed with GO to form a GO/aptamer complex, and the feasibility of the complex was tested in vitro. The high correlation between the fluorescence intensity and concentration of ATP was observed in the range 0–10 mM. Next, the feasibility of the complex was confirmed in vivo. Under both phototrophic and heterotrophic culture conditions, Euglena gracilis internalized the complex, and bright fluorescence was observed as the aptamer was bound to the target metabolite (ATP). The fluorescence intensity of cells was correlated to the ATP concentration in the cells. Imaging of dual intracellular metabolites (ATP and paramylon) was achieved by simply using two different aptamers (ATP-specific aptamer and paramylon-specific aptamer) together, showing the great potential of the complex as a dual-sensing/imaging platform. In addition, the GO/aptamer complex exhibited low cytotoxicity; the proliferation and viability of E. gracilis cells were not significantly affected by the complex. Our results suggested that this new imaging platform can be efficiently used for detecting dual intracellular metabolites in live microalgal cells.
Graphical abstract







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Shimizu Y (1993) Microalgal metabolites. Chem Rev 93(5):1685–1698
Mu N, Mehar JG, Mudliar SN et al (2019) Recent advances in microalgal bioactives for food, feed, and healthcare products: commercial potential, market space, and sustainability. Compr Rev Food Sci Food Saf 18(6):1882–1897
Elisabeth B, Rayen F, Behnam T (2021) Microalgae culture quality indicators: a review. Crit Rev Biotechnol 41(4):457–473
Kim JY, Oh JJ, Kim DH et al (2019) Rapid and accurate quantification of paramylon produced from Euglena gracilis using an ssDNA aptamer. J Agric Food Chem 68(1):402–408
Wakisaka Y, Suzuki Y, Iwata O et al (2016) Probing the metabolic heterogeneity of live Euglena gracilis with stimulated Raman scattering microscopy. Nat Microbiol 1(10):1–4
Muñoz HE, Li M, Riche CT et al (2018) Single-cell analysis of morphological and metabolic heterogeneity in Euglena gracilis by fluorescence-imaging flow cytometry. Anal Chem 90(19):11280–11289
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346(6287):818–822
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: rna ligands to bacteriophage T4 DNA polymerase. Science 249(4968):505–510
Keefe AD, Pai S, Ellington A (2010) Aptamers as therapeutics. Nat Rev Drug Discov 9(7):537–550
Dunn MR, Jimenez RM, Chaput JC (2017) Analysis of aptamer discovery and technology. Nat Rev Chem 1(10):1–16
Espiritu CAL, Justo CAC, Rubio MJ et al (2018) aptamer selection against a Trichomonas vaginalis adhesion protein for diagnostic applications. ACS Infect Dis 4(9):1306–1315
Huizenga DE, Szostak JW (1995) A DNA aptamer that binds adenosine and ATP. Biochemistry 34(2):656–665
Ng A, Chinnappan R, Eissa S et al (2012) Selection, characterization, and biosensing application of high affinity congener-specific microcystin-targeting aptamers. Environ Sci Technol 46(19):10697–10703
Wang Y, Li Z, Weber TJ et al (2013) In situ live cell sensing of multiple nucleotides exploiting DNA/RNA aptamers and graphene oxide nanosheets. Anal Chem 85(14):6775–6782
Wang Y, Tang L, Li Z et al (2014) In situ simultaneous monitoring of ATP and GTP using a graphene oxide nanosheet–based sensing platform in living cells. Nat Protoc 9(8):1944
Wang Y, Li Z, Hu D et al (2010) Aptamer/graphene oxide nanocomplex for in situ molecular probing in living cells. J Am Chem Soc 132(27):9274–9276
Tan X, Chen T, Xiong X et al (2012) Semiquantification of ATP in live cells using nonspecific desorption of dna from graphene oxide as the internal reference. Anal Chem 84(20):8622–8627
Liu Z, Chen S, Liu B et al (2014) Intracellular detection of ATP using an aptamer beacon covalently linked to graphene oxide resisting nonspecific probe displacement. Anal Chem 86(24):12229–12235
Wan LY, Yuan WF, Ai WB et al (2019) An exploration of aptamer internalization mechanisms and their applications in drug delivery. Expert Opin Drug Deliv 16(3):207–218
Bouvier-Müller A, Ducongé F (2018) Application of aptamers for in vivo molecular imaging and theranostics. Adv Drug Deliv Rev 134:94–106
Bamburowicz-Klimkowska M, Poplawska M, Grudzinski IP (2019) Nanocomposites as biomolecules delivery agents in nanomedicine. J Nanobiotechnol 17(1):48
Ebrahimi SB, Samanta D, Mirkin CA (2020) DNA-based nanostructures for live-cell analysis. J Am Chem Soc 142(26):11343–11356
Lu CH, Yang HH, Zhu CL et al (2009) A graphene platform for sensing biomolecules. Angew Chem Int Edit 121(26):4879–4881
Yang L, Liu B, Wang M et al (2018) A highly sensitive strategy for fluorescence imaging of microrna in living cells and in vivo based on graphene oxide-enhanced signal molecules quenching of molecular beacon. ACS Appl Mater Interfaces 10(8):6982–6990
Lopez A, Liu J (2020) Covalent and noncovalent functionalization of graphene oxide with DNA for smart sensing. Adv Intell Syst 2(11):2000123
Huang B, Miao AJ, Xiao L et al (2017) Influence of nitrogen limitation on the bioaccumulation kinetics of hematite nanoparticles in the freshwater alga Euglena intermedia. Environ Sci Nano 4(9):1840–1850
Lee WM, Yoon SJ, Shin YJ et al (2015) Trophic transfer of gold nanoparticles from Euglena gracilis or Chlamydomonas reinhardtii to Daphnia magna. Environ Pollut 201:10–16
Jung JM, Kim JY, Jung S et al (2021) Quantitative study on lipid productivity of Euglena gracilis and its biodiesel production according to the cultivation conditions. J Clean Prod 291:125218
Kong RM, Zhang XB, Chen Z et al (2011) Aptamer-assembled nanomaterials for biosensing and biomedical applications. Small 7(17):2428–2436
Dong Y, Zhang T, Lin X et al (2020) Graphene/aptamer probes for small molecule detection: from in vitro test to in situ imaging. Microchim Acta 187(3):1–18
Liu J, Cui L, Losic D (2013) Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater 9(12):9243–9257
Wang Y, Li Z, Wang J et al (2011) Graphene and graphene oxide: biofunctionalization and applications in biotechnology. Trends Biotechnol 29(5):205–212
Ali-Boucetta H, Bitounis D, Raveendran-Nair R et al (2013) Purified graphene oxide dispersions lack in vitro cytotoxicity and in vivo pathogenicity. Adv Healthc Mater 2:433–441
Zhang Z, Oni O, Liu J (2017) New insights into a classic aptamer: binding sites, cooperativity and more sensitive adenosine detection. Nucleic Acids Res 45(13):7593–7601
Chang H, Tang L, Wang Y et al (2010) Graphene fluorescence resonance energy transfer aptasensor for the thrombin detection. Anal Chem 82(6):2341–2346
Li X, Schirmer K, Bernard L et al (2015) silver nanoparticle toxicity and association with the alga Euglena gracilis. Environ Sci Nano 2(6):594–602
Mu Q, Su G, Li L et al (2012) Size-dependent cell uptake of protein-coated graphene oxide nanosheets. ACS Appl Mater Interfaces 4(4):2259–2266
Wang Y, Li Z, Liu M (2017) Multiple-targeted graphene-based nanocarrier for intracellular imaging of mRNAs. Anal Chim Acta 983:1–8
Zhang H, Peng C, Yang J et al (2013) Uniform ultrasmall graphene oxide nanosheets with low cytotoxicity and high cellular uptake. ACS Appl Mater Interfaces 5(5):1761–1767
Conner SD, Schmid SL (2003) Regulated portals of entry into the cell. Nature 422(6927):37–44
Tu Z, Achazi K, Schulz A et al (2017) Combination of surface charge and size controls the cellular uptake of functionalized graphene sheets. Adv Funct Mater 27(33):1701837
Fink BD, Bai F, Yu L et al (2017) Regulation of ATP production: dependence on calcium concentration and respiratory state. Am J Physiol Cell Physiol 313:C146–C153
Weber SC, Spakowitz AJ, Theriot JA (2012) Nonthermal ATP-dependent fluctuations contribute to the in vivo motion of chromosomal loci. Proc Natl Acad Sci 109(19):7338–7343
Ota N, Yonamine Y, Asai T et al (2019) Isolating single Euglena gracilis cells by glass microfluidics for Raman analysis of paramylon biogenesis. Anal Chem 91(15):9631–9639
Maeno T, Uzawa T, Kono I et al (2018) Targeted delivery of fluorogenic peptide aptamers into live microalgae by femtosecond laser photoporation at single-cell resolution. Sci Rep 8(1):1–9
Seabra AB, Paula AJ, de Lima R et al (2014) Nanotoxicity of graphene and graphene oxide. Chem Res Toxicol 27(2):159–168
Akhavan O, Ghaderi E (2010) Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 4(10):5731–5736
Hu C, Wang Q, Zhao H et al (2015) Ecotoxicological effects of graphene oxide on the protozoan Euglena gracilis. Chemosphere 128:184–190
Nakano Y, Urade Y, Urade R et al (1987) Isolation, purification, and characterization of the pellicle of Euglena gracilis Z. J Biochem 102(5):1053–1063
Kim JY, Oh JJ, Kim HS et al (2021) Application of electrical treatment on euglena gracilis for increasing paramylon production. Appl Microbiol Biotechnol 105:1031–1039
Jang SC, Kang SM, Lee JY et al (2018) Nano-graphene oxide composite for in vivo imaging. Int J Nanomed 13:221
Lu CH, Zhu CL, Li J et al (2010) Using graphene to protect DNA from cleavage during cellular delivery. Chem Commun 46(18):3116–3118
Hu X, Lu K, Mu L et al (2014) Interactions between graphene oxide and plant cells: regulation of cell morphology, uptake, organelle damage, oxidative effects and metabolic disorders. Carbon 80:665–676
Li M, Muñoz HE, Goda K et al (2017) Shape-based separation of microalga euglena gracilis using inertial microfluidics. Sci Rep 7(1):1–8
Funding
This study was supported by an NRF (National Research Foundation of Korea) grant funded by the Korean Government (MSIT; 2019R1A2C2087449, NRF-2021R1A6A1A10045235, NRF-2021R1F1A1063455, and NRF-2018-Global Ph. D. Fellowship program). This research was also supported by Korea Environment Industry & Technology Institute (KEITI) through Ecological Imitation-based Environmental Pollution Management Technology Development Project, funded by the Korea Ministry Environment (MOE) (2019002790009).
Author information
Authors and Affiliations
Contributions
J.Y.K designed the study, carried out the experiments, and wrote the manuscript. C.R.J characterized materials. J.P contributed to data analysis and designed the figures. D.G.K contributed to data analysis. H.S.K and Y.E.C supervised the experiments and revised the manuscript. All authors in this study reviewed the manuscript and commented on the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This paper does not contain any studies with humans or animals.
Consent for publication
All authors are consent for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, J.Y., Jin, C.R., Park, J. et al. Simultaneous probing of dual intracellular metabolites (ATP and paramylon) in live microalgae using graphene oxide/aptamer nanocomplex. Microchim Acta 189, 88 (2022). https://doi.org/10.1007/s00604-022-05198-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05198-5