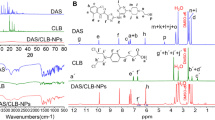
Abstract
Near-infrared fluorescent (NIRF) dye-coupled self-assembled RGD-linked proapoptotic peptide nanoparticles have been synthesized with spherical shape and size ~ 30–40 nm diameters. The peptide sequence was coupled with cyanine 5.5 probe as NIRF-dye to introduce optical imaging properties and pH-dependent method was used to design Cy5.5 coupled self-assembled peptide nanoparticles (f-SAPNs). This nanoprobe has the ability to target αvβ3-integrin receptor overexpressed on cancer cell’s surface with improved internalization capabilities into the mitochondria. The in situ study showed that this peptide sequence has potential to disrupt the mitochondrial membrane efficiently, activating the Caspase-3 enzyme, and ultimately induces cell apoptosis. It has been observed from in vitro study that the degree of apoptosis for f-SAPNs was increased from 25.6% to 96.3%, while decreased degree of necrosis from 51.7% to 0.2% compared with its parent peptide analog (Cy5.5-c[RGDKLAK]; f-CP) occurs. Further investigations revealed that these f-SAPNs showed high uptake in U87MG glioblastoma cells in comparison with PC-3 prostate cancer cells. Moreover, in vivo therapeutic studies represented the prominent decrease in the size of tumor tissue treated with f-CP and f-SAPNs (201 ± 13 mm3 and 104 ± 6 mm3, respectively) compared with untreated tumor tissues (366 ± 18 mm3). These outcomes highlighted the specificity, and efficacy of f-SAPNs toward αvβ3-integrin expressing tumor tissue in vivo and suggested that these novel designed f-SAPNs may serve as a potential theranostic drug for brain tumor glioblastoma multiforme.
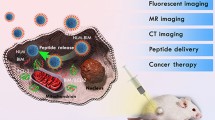
Graphical abstract
The pH-sensitive method gives NIRF dye-coupled self-assembled peptide nanoparticle (f-SAPNs), enables the tunable synthesis of spherical nanoparticles with high stability towards proteolysis, improved biocompatibility, and promising therapeutic efficacy.








Similar content being viewed by others
References
Askari Rizvi SF, Zhang H (2021) Emerging trends of receptor-mediated tumor targeting peptides: A review with perspective from molecular imaging modalities. Eur J Med Chem 221:113538–113555. https://doi.org/10.1016/j.ejmech.2021.113538
Porru M, Zappavigna S, Salzano G, Luce A, Stoppacciaro A, Balestrieri ML, Artuso S, Lusa S, De Rosa G, Leonetti C, Caraglia M (2014) Medical treatment of orthotopic glioblastoma with transferrin-conjugated nanoparticles encapsulating zoledronic acid. Oncotarget. 5:10446–10459. https://doi.org/10.18632/oncotarget.2182
Reja SI, Minoshima M, Hori Y, Kikuchi K (2021) Near-infrared fluorescent probes: a next-generation tool for protein-labeling applications. Chem Sci 12:3437–3447. https://doi.org/10.1039/d0sc04792a
He L, Tan C-P, Ye R-R, Zhao Y-Z, Liu Y-H, Zhao Q, Ji L-N, Mao Z-W (2014) Theranostic iridium(III) complexes as one- and two-photon phosphorescent trackers to monitor autophagic lysosomes. Angew Chem Int Ed 53:12137–12141. https://doi.org/10.1002/anie.201407468
Yang Z, Lee JH, Jeon HM, Han JH, Park N, He Y, Lee H, Hong KS, Kang C, Kim JS (2013) Folate-based near-infrared fluorescent theranostic gemcitabine delivery. J Am Chem Soc 135:11657–11662. https://doi.org/10.1021/ja405372k
Kumar R, Han J, Lim H-J, Ren WX, Lim J-Y, Kim J-H, Kim JS (2014) Mitochondrial induced and self-monitored intrinsic apoptosis by antitumor theranostic prodrug: in vivo imaging and precise cancer treatment. J Am Chem Soc 136:17836–17843. https://doi.org/10.1021/ja510421q
Zhang X, Ba Q, Gu Z, Guo D, Zhou Y, Xu Y, Wang H, Ye D, Liu H (2015) Fluorescent coumarin–artemisinin conjugates as mitochondria-targeting theranostic probes for enhanced anticancer activities. Chem-A Eur J 21:17415–17421. https://doi.org/10.1002/chem.201502543
Hu Q, Gao M, Feng G, Liu B (2014) Mitochondria-targeted cancer therapy using a light-up probe with aggregation-induced-emission characteristics. Angew Chem Int Ed 53:14225–14229. https://doi.org/10.1002/anie.201408897
Gao P, Pan W, Li N, Tang B (2019) Fluorescent probes for organelle-targeted bioactive species imaging. Chem Sci 10:6035–6071. https://doi.org/10.1039/c9sc01652j
Wisnovsky S, Jean SR, Liyanage S, Schimmer A, Kelley SO (2016) Mitochondrial DNA repair and replication proteins revealed by targeted chemical probes. Nat Chem Biol 12:567–573. https://doi.org/10.1038/nchembio.2102
Sun T, Guan X, Zheng M, Jing X, Xie Z (2015) Mitochondria-localized fluorescent BODIPY-platinum conjugate. ACS Med Chem Lett 6:430–433. https://doi.org/10.1021/acsmedchemlett.5b00041
Khan MM, Filipczak N, Torchilin VP (2021) Cell penetrating peptides: a versatile vector for co-delivery of drug and genes in cancer. J Control Release 330:1220–1228. https://doi.org/10.1016/j.jconrel.2020.11.028
Suma T, Cui J, Müllner M, Fu S, Tran J, Noi KF, Ju Y, Caruso F (2017) Modulated fragmentation of proapoptotic peptide nanoparticles regulates cytotoxicity. J Am Chem Soc 139:4009–4018. https://doi.org/10.1021/jacs.6b11302
Kim HY, Kim S, Youn H, Chung J-K, Shin DH, Lee K (2011) The cell penetrating ability of the proapoptotic peptide, KLAKLAKKLAKLAK fused to the N-terminal protein transduction domain of translationally controlled tumor protein. MIIYRDLISH Biomaterials 32:5262–5268. https://doi.org/10.1016/j.biomaterials.2011.03.074
Nieberler M, Reuning U, Reichart F, Notni J, Wester H-J, Schwaiger M, Weinmüller M, Räder A, Steiger K, Kessler H (2017) Exploring the role of RGD-recognizing integrins in cancer. Cancers 9:116–125. https://doi.org/10.3390/cancers9090116
Hou J, Diao Y, Li W, Yang Z, Zhang L, Chen Z, Wu Y (2016) RGD peptide conjugation results in enhanced antitumor activity of PD0325901 against glioblastoma by both tumor-targeting delivery and combination therapy. Int J Pharm 505:329–340. https://doi.org/10.1016/j.ijpharm.2016.04.017
Zhang P, Cui Y, Anderson CF, Zhang C, Li Y, Wang R, Cui H (2018) Peptide-based nanoprobes for molecular imaging and disease diagnostics. Chem Soc Rev 47:3490–3529. https://doi.org/10.1039/c7cs00793k
Cheng K, Ding Y, Zhao Y, Ye S, Zhao X, Zhang Y, Ji T, Wu H, Wang B, Anderson GJ, Ren L, Nie G (2018) Sequentially responsive therapeutic peptide assembling nanoparticles for dual-targeted cancer immunotherapy. Nano Lett 18:3250–3258. https://doi.org/10.1021/acs.nanolett.8b01071
Wang W, Chau Y (2012) Self-assembly mediated platform for rapid and facile preparation of peptide-functionalized nanoparticles with high stability. Chem Mater 24:946–953. https://doi.org/10.1021/cm202860h
Rizvi SFA, Mu S, Wang Y, Li S, Zhang H (2020) Fluorescent RGD-based pro-apoptotic peptide conjugates as mitochondria-targeting probes for enhanced anticancer activities. Biomed Pharmacother 127:110179–110189. https://doi.org/10.1016/j.biopha.2020.110179
Kong J, Zhang J, Wang Y, Qi W, Rao H, Hu L, Su R, He Z (2020) Bioinspired pH-sensitive fluorescent peptidyl nanoparticles for cell imaging. ACS Appl Mater Interfaces 12:4212–4220. https://doi.org/10.1021/acsami.9b17866
Pace CN, Scholtz JM (1998) A helix propensity scale based on experimental studies of peptides and proteins. Biophys J 75:422–427. https://doi.org/10.1016/s0006-3495(98)77529-0
Bujacz A (2012) Structures of bovine, equine and leporine serum albumin. Acta Crystallogr D Biol Crystallogr 68:1278–1289. https://doi.org/10.1107/s0907444912027047
Yeggoni DP, Gokara M, Mark Manidhar D, Rachamallu A, Nakka S, Reddy CS, Subramanyam R (2014) Binding and molecular dynamics studies of 7-hydroxycoumarin derivatives with human serum albumin and its pharmacological importance. Mol Pharm 11:1117–1131. https://doi.org/10.1021/mp500051f
Parodi A, Corbo C, Cevenini A, Molinaro R, Palomba R, Pandolfi L, Agostini M, Salvatore F, Tasciotti E (2015) Enabling cytoplasmic delivery and organelle targeting by surface modification of nanocarriers. Nanomedicine (Lond) 10:1923–1940. https://doi.org/10.2217/nnm.15.39
Ye Y, Zhang T, Yuan H, Li D, Lou H, Fan P (2017) Mitochondria-targeted lupane triterpenoid derivatives and their selective apoptosis-inducing anticancer mechanisms. J Med Chem 60:6353–6363. https://doi.org/10.1021/acs.jmedchem.7b00679
Fan Z, Chang Y, Cui C, Sun L, Wang DH, Pan Z, Zhang M (2018) Near infrared fluorescent peptide nanoparticles for enhancing esophageal cancer therapeutic efficacy. Nat Commun 9:2605–2617. https://doi.org/10.1038/s41467-018-04763-y
Qiao ZY, Lin YX, Lai WJ, Hou CY, Wang Y, Qiao SL, Zhang D, Fang QJ, Wang H (2016) A general strategy for facile synthesis and in situ screening of self-assembled polymer-peptide nanomaterials. Adv Mater 28:1859–1867. https://doi.org/10.1002/adma.201504564
Liu F-H, Hou C-Y, Zhang D, Zhao W-J, Cong Y, Duan Z-Y, Qiao Z-Y, Wang H (2018) Enzyme-sensitive cytotoxic peptide–dendrimer conjugates enhance cell apoptosis and deep tumor penetration. Biomaterials Sci 6:604–613. https://doi.org/10.1039/c7bm01182b
Vázquez O, Seitz O (2014) Cytotoxic peptide–PNA conjugates obtained by RNA-programmed peptidyl transfer with turnover. Chem Sci 5:2850–2854. https://doi.org/10.1039/c4sc00299g
Sun L, Fan Z, Wang Y, Huang Y, Schmidt M, Zhang M (2015) Tunable synthesis of self-assembled cyclic peptide nanotubes and nanoparticles. Soft Matter 11:3822–3832. https://doi.org/10.1039/c5sm00533g
Ruoslahti E, Bhatia SN, Sailor MJ (2010) Targeting of drugs and nanoparticles to tumors. J Cell Biol 188:759–768. https://doi.org/10.1083/jcb.200910104
Zhao Y, Ji T, Wang H, Li S, Zhao Y, Nie G (2014) Self-assembled peptide nanoparticles as tumor microenvironment activatable probes for tumor targeting and imaging. J Control Release 177:11–19. https://doi.org/10.1016/j.jconrel.2013.12.037
Ngambenjawong C, Gustafson HH, Pineda JM, Kacherovsky NA, Cieslewicz M, Pun SH (2016) Serum stability and affinity optimization of an M2 macrophage-targeting peptide (M2pep). Theranostics 6:1403–1414. https://doi.org/10.7150/thno.15394
Suvarna M, Dyawanapelly S, Kansara B, Dandekar P, Jain R (2018) Understanding the stability of nanoparticle–protein interactions: effect of particle size on adsorption, conformation and thermodynamic properties of serum albumin proteins. ACS Applied Nano Materials 1:5524–5535. https://doi.org/10.1021/acsanm.8b01019
Parsekar SU, Velankanni P, Sridhar S, Haldar P, Mate NA, Banerjee A, Sudhadevi Antharjanam PK, Koley AP, Kumar M (2020) Protein binding studies with human serum albumin, molecular docking and in vitro cytotoxicity studies using HeLa cervical carcinoma cells of Cu(ii)/Zn(ii) complexes containing a carbohydrazone ligand. Dalton Trans 49:2947–2965. https://doi.org/10.1039/c9dt04656a
González-Béjar M, Alarcón E, Poblete H, Scaiano JC, Pérez-Prieto J (2010) Stereoselective interaction of epimeric naproxen-RGD peptides with human serum albumin. Biomacromol 11:2255–2260. https://doi.org/10.1021/bm100808d
Gao Y, Huang J, Zhou Q, Liu R, Zhang S, Zhang C, Huang Y-Y, Li Z, Huang L, Wu D, Wu Y, Xiao L, Guo L, Luo H-B (2021) Discovery of highly specific catalytic-site-targeting fluorescent probes for detecting lysosomal PDE10A in living cells. ACS Chem Biol 16:857–863. https://doi.org/10.1021/acschembio.1c00018
Kang MH, Reynolds CP (2009) Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clinical cancer research : an official journal of the American Association for Cancer Research 15:1126–1132. https://doi.org/10.1158/1078-0432.ccr-08-0144
Cai G, Yu W, Song D, Zhang W, Guo J, Zhu J, Ren Y, Kong L (2019) Discovery of fluorescent coumarin-benzo[b]thiophene 1, 1-dioxide conjugates as mitochondria-targeting antitumor STAT3 inhibitors. Eur J Med Chem 174:236–251. https://doi.org/10.1016/j.ejmech.2019.04.024
Levasseur MD, Mantri S, Hayashi T, Reichenbach M, Hehn S, Waeckerle-Men Y, Johansen P, Hilvert D (2021) Cell-specific delivery using an engineered protein nanocage. ACS Chem Biol 16:838–843. https://doi.org/10.1021/acschembio.1c00007
Clementino A, Velasco-Estevez M, Buttini F, Sonvico F, Dev KK (2021) Hybrid nanoparticles as a novel tool for regulating psychosine-induced neuroinflammation and demyelination in vitro and ex vivo. Neurotherapeutics 9:1–15. https://doi.org/10.1007/s13311-021-01109-3
AshaRani PV, Low KahMun G, Hande MP, Valiyaveettil S (2009) Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 3:279–290. https://doi.org/10.1021/nn800596w
Huang CW, Li Z, Conti PS (2011) In vivo near-infrared fluorescence imaging of integrin alpha2beta1 in prostate cancer with cell-penetrating-peptide-conjugated DGEA probe. J Nucl Med 52:1979–1986. https://doi.org/10.2967/jnumed.111.091256
Funding
This research work was financially supported by the National Natural Science Foundation of China (No. 21575055).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All animal studies performed in this study were in accordance with the compliance of animal ethical committee of the National Institute of Health and National Regulation of China for Care and Use of Laboratory Animals (Lanzhou University, Gansu, China).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
604_2021_5148_MOESM1_ESM.docx
Supplementary file1 (DOCX 5099 KB) The online version of supplementary material contains description about characterizations, protocols for in vitro cell studies, in silico study, and therapeutic study available (Pdf).
Rights and permissions
About this article
Cite this article
Rizvi, S.F.A., Mu, S., Zhao, C. et al. Fabrication of self-assembled peptide nanoparticles for in vitro assessment of cell apoptosis pathway and in vivo therapeutic efficacy. Microchim Acta 189, 53 (2022). https://doi.org/10.1007/s00604-021-05148-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-05148-7