Abstract
Change in the level of human prostate-specific antigen (PSA) is a major element in the development and progression of prostate cancer (PCa). Most of the methodologies are currently restricted to their application in routine clinical screening due to the scarcity of adequate screening tools, false reading, long assay time, and cost. Innovative techniques and the integration of knowledge from a variety of domains, such as materials science and engineering, are needed to provide sustainable solutions. The convergence of precision point-of-care (POC) diagnostic techniques, which allow patients to respond in real time to changes in PSA levels, provides promising possibilities for quantitative and quantitative detection of PSA. This solution could be interesting and relevant for use in PCa diagnosis at the POC. The approaches enable low-cost real-time detection and are simple to integrate into user-friendly sensor devices. This review focuses on the investigations, prospects, and challenges associated with integrating engineering sciences with cancer biology to develop nanotechnology-based tools for PCa diagnosis. This article intends to encourage the development of new nanomaterials to construct high-performance POC devices for PCa detection. Finally, the review concludes with closing remarks and a perspective forecast.
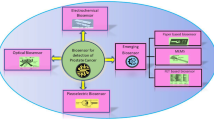
Graphical abstract








Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin. https://doi.org/10.3322/caac.21442
Taitt HE (2018) Global trends and prostate cancer: a review of incidence, detection, and mortality as influenced by race, ethnicity, and geographic location. Am. J. Mens. Health
Damborska D, Bertok T, Dosekova E, et al (2017) Nanomaterial-based biosensors for detection of prostate specific antigen. Microchim. Acta
Tangel MR, Rastinehad AR (2018) Advances in prostate cancer imaging. F1000Research. https://doi.org/10.12688/f1000research.14498.1
Fuchsjager M, Shukla-Dave A, Akin O et al (2008) Prostate cancer imaging. Acta radiol. https://doi.org/10.1080/02841850701545821
van Luijtelaar A, Bomers J, Fütterer J (2019) A comparison of magnetic resonance imaging techniques used to secure biopsies in prostate cancer patients. Expert Rev. Anticancer Ther.
Korevaar S, Tennakoon R, Page M et al (2021) Incidental detection of prostate cancer with computed tomography scans. Sci Rep. https://doi.org/10.1038/s41598-021-86972-y
Ghai S, Toi A (2012) Role of transrectal ultrasonography in prostate cancer. Radiol. Clin. North Am.
Smeenge M, Barentsz J, Cosgrove D, et al (2012) Role of transrectal ultrasonography (TRUS) in focal therapy of prostate cancer: report from a Consensus Panel. BJU Int.
Zimmerman ME, Meyer AR, Rowe SP, Gorin MA (2019) Imaging of prostate cancer with positron emission tomography. Clin Adv Hematol Oncol
Lütje S, Boerman OC, Van Rij CM, et al (2012) Prospects in radionuclide imaging of prostate cancer. Prostate
Ferraro DA, Burger IA (2020) Prostate cancer: prostate-specific membrane antigen positron-emission tomography/computed tomography or positron-emission tomography/magnetic resonance imaging for staging. Top. Magn. Reson. Imaging
Catalona WJ, Ratliff TL, Dodds KM et al (1991) Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. https://doi.org/10.1056/NEJM199104253241702
Jones AL, Dhanapala L, Baldo TA et al (2021) Prostate cancer diagnosis in the clinic using an 8-protein biomarker panel. Anal Chem. https://doi.org/10.1021/acs.analchem.0c04034
Stamey TA, Yang N, Hay AR et al (1987) Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. https://doi.org/10.1056/nejm198710083171501
Barisiene M, Bakavicius A, Stanciute D et al (2020) Prostate Health Index and Prostate Health Index Density as diagnostic tools for improved prostate cancer detection. Biomed Res Int. https://doi.org/10.1155/2020/9872146
Borghesi M, Ahmed H, Nam R, et al (2017) Complications after systematic, random, and image-guided prostate biopsy [figure presented]. Eur. Urol.
Heydari-Bafrooei E, Shamszadeh NS (2017) Electrochemical bioassay development for ultrasensitive aptasensing of prostate specific antigen. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2016.12.048
Arya SK, Bhansali S (2011) Lung cancer and its early detection using biomarker-based biosensors. Chem. Rev.
Primiceri E, Chiriacò MS, Notarangelo FM, et al (2018) Key enabling technologies for point-of-care diagnostics. Sensors (Switzerland)
Cinti S, Moscone D, Arduini F (2019) Preparation of paper-based devices for reagentless electrochemical (bio) sensor strips. Nat Protoc. https://doi.org/10.1038/s41596019-0186-y
Parolo C, Sena-Torralba A, Bergua JF, Calucho E, Fuentes-Chust C, Hu L, Rivas L, Álvarez-Diduk R, Nguyen EP, Cinti S, Quesada-González D, Merkoçi A (2020) Tutorial: design and fabrication of nanoparticle-based lateral-flow immunoassays. Nat Protoc. https://doi.org/10.1038/s41596-020-0357-x
Traynor SM, Pandey R, Maclachlan R et al (2020) Review—recent advances in electrochemical detection of prostate specific antigen (PSA) in clinically-relevant samples. J Electrochem Soc. https://doi.org/10.1149/1945-7111/ab69fd
Ortone V, Matino L, Santoro F, Cinti S (2021) Merging office/filter paper-based tools for pre-concentring and detecting heavy metals in drinking water. Chem Commun. https://doi.org/10.1039/D1CC02481G
Cinti S, Marrone R, Mazzaracchio V, Moscone D, Arduini F (2020) Novel bio-lab-on-a tip for electrochemical glucose sensing in commercial beverages. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2020.112334
Singh S, Numan A, Cinti S (2021) Point-of-care for evaluating antimicrobial resistance through the adoption of functional materials. Anal Chem. https://doi.org/10.1021/acs.analchem.1c03856
Singh S, Gill AAS, Nlooto M, Karpoormath R (2019) Prostate cancer biomarkers detection using nanoparticles based electrochemical biosensors. Biosens. Bioelectron.
Tajik S, Dourandish Z, Jahani PM et al (2021) Recent developments in voltammetric and amperometric sensors for cysteine detection. RSC Adv. https://doi.org/10.1039/d0ra07614g
Jalalvand AR (2019) Fabrication of a novel and ultrasensitive label-free electrochemical aptasensor for detection of biomarker prostate specific antigen. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2019.01.012
Li H, Wei Q, Wang G et al (2011) Sensitive electrochemical immunosensor for cancer biomarker with signal enhancement based on nitrodopamine-functionalized iron oxide nanoparticles. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2010.12.011
Yazdani Z, Yadegari H, Heli H (2019) A molecularly imprinted electrochemical nanobiosensor for prostate specific antigen determination. Anal Biochem. https://doi.org/10.1016/j.ab.2018.11.020
Li F, Li Y, Feng J et al (2017) Ultrasensitive amperometric immunosensor for PSA detection based on Cu2O@CeO2-Au nanocomposites as integrated triple signal amplification strategy. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2016.09.018
Bhansali S, Chornokur G, Arya SK et al (2011) Impedance-based miniaturized biosensor for ultrasensitive and fast prostate-specific antigen detection. J Sensors. https://doi.org/10.1155/2011/983752
Perfézou M, Turner A, Merkoçi A (2012) Cancer detection using nanoparticle-based sensors. Chem. Soc. Rev.
Ouhibi A, Raouafi A, Lorrain N et al (2021) Functionalized SERS substrate based on silicon nanowires for rapid detection of prostate specific antigen. Sensors Actuators B Chem. https://doi.org/10.1016/j.snb.2020.129352
Lin S, Zhong J, Chi Y et al (2021) Colorimetric immunosensor based on glassy carbon microspheres test strips for the detection of prostate-specific antigen. Microchim Acta. https://doi.org/10.1007/s00604-021-04907-w
Xia N, Deng D, Wang Y et al (2018) Gold nanoparticle-based colorimetric method for the detection of prostate-specific antigen. Int J Nanomedicine. https://doi.org/10.2147/IJN.S154046
Karami P, Khoshsafar H, Johari-Ahar M et al (2019) Colorimetric immunosensor for determination of prostate specific antigen using surface plasmon resonance band of colloidal triangular shape gold nanoparticles. Spectrochim Acta - Part A Mol Biomol Spectrosc. https://doi.org/10.1016/j.saa.2019.117218
Kong RM, Zhang X, Ding L et al (2017) Label-free fluorescence turn-on aptasensor for prostate-specific antigen sensing based on aggregation-induced emission–silica nanospheres. Anal Bioanal Chem. https://doi.org/10.1007/s00216-017-0519-z
Nxele SR, Nyokong T (2021) The electrochemical detection of prostate specific antigen on glassy carbon electrode modified with combinations of graphene quantum dots, cobalt phthalocyanine and an aptamer. J Inorg Biochem. https://doi.org/10.1016/j.jinorgbio.2021.111462
Ibau C, Md Arshad MK, Subash CBG et al (2019) Gold interdigitated triple-microelectrodes for label-free prognosticative aptasensing of prostate cancer biomarker in serum. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2019.04.048
Suresh L, Brahman PK, Reddy KR, Bondili JS (2018) Development of an electrochemical immunosensor based on gold nanoparticles incorporated chitosan biopolymer nanocomposite film for the detection of prostate cancer using PSA as biomarker. Enzyme Microb Technol. https://doi.org/10.1016/j.enzmictec.2017.10.009
Rafique S, Bin W, Bhatti AS (2015) Electrochemical immunosensor for prostate-specific antigens using a label-free second antibody based on silica nanoparticles and polymer brush. Bioelectrochemistry. https://doi.org/10.1016/j.bioelechem.2014.08.001
Wang H, Zhang Y, Yu H et al (2013) Label-free electrochemical immunosensor for prostate-specific antigen based on silver hybridized mesoporous silica nanoparticles. Anal Biochem. https://doi.org/10.1016/j.ab.2012.11.012
Mahani M, Alimohamadi F, Torkzadeh-Mahani M et al (2021) LSPR biosensing for the early-stage prostate cancer detection using hydrogen bonds between PSA and antibody: molecular dynamic and experimental study. J Mol Liq. https://doi.org/10.1016/j.molliq.2020.114736
Khan Y, Li A, Chang L et al (2018) Gold nano disks arrays for localized surface plasmon resonance based detection of PSA cancer marker. Sensors Actuators B Chem. https://doi.org/10.1016/j.snb.2017.08.118
Zhang J, Wang S, Gao N et al (2015) Luminescence energy transfer detection of PSA in red region based on Mn2+-enhanced NaYF4:YB, Er upconversion nanorods. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2015.05.024
Cai Y, Zhang S, Dong C et al (2021) Lateral flow immunoassay based on gold magnetic nanoparticles for the protein quantitative detection: Prostate-specific antigen. Anal Biochem. https://doi.org/10.1016/j.ab.2021.114265
Shayesteh OH, Ghavami R (2020) A novel label-free colorimetric aptasensor for sensitive determination of PSA biomarker using gold nanoparticles and a cationic polymer in human serum. Spectrochim Acta - Part A Mol Biomol Spectrosc. https://doi.org/10.1016/j.saa.2019.117644
Smith SJ, Nemr CR, Kelley SO (2017) Chemistry-driven approaches for ultrasensitive nucleic acid detection. J. Am. Chem. Soc.
Subramani K, Elhissi A, Subbiah U, Ahmed W (2019) Introduction to nanotechnology. In: Nanobiomaterials in Clinical Dentistry
Farshchi F, Hasanzadeh M (2020) Nanomaterial based aptasensing of prostate specific antigen (PSA): Recent progress and challenges in efficient diagnosis of prostate cancer using biomedicine. Biomed Pharmacother. https://doi.org/10.1016/j.biopha.2020.110878
Robbs PH, Rees NV (2016) Nanoparticle electrochemistry. Phys Chem Chem Phys. https://doi.org/10.1039/c6cp05101d
Zheng G, Patolsky F, Cui Y et al (2005) Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat Biotechnol. https://doi.org/10.1038/nbt1138
Xu L, Wen Y, Pandit S, et al (2019) Graphene-based biosensors for the detection of prostate cancer protein biomarkers: a review. BMC Chem.
Novoselov KS, Geim AK, Morozov S V., et al (2004) Electric field in atomically thin carbon films. Science (80). https://doi.org/10.1126/science.1102896
Prattis I, Hui E, Gubeljak P, et al (2021) Graphene for biosensing applications in point-of-care testing. Trends Biotechnol.
Yang W, Ratinac KR, Ringer SR, et al (2010) Carbon nanomaterials in biosensors: should you use nanotubes or graphene. Angew. Chemie - Int. Ed.
Zheng G, Lieber CM (2011) Nanowire biosensors for label-free, real-time, ultrasensitive protein detection. Methods Mol Biol. https://doi.org/10.1007/978-1-61779-319-6_18
Banks CE, Davies TJ, Wildgoose GG, Compton RG (2005) Electrocatalysis at graphite and carbon nanotube modified electrodes: Edge-plane sites and tube ends are the reactive sites. Chem. Commun.
Lu J, Liu S, Ge S et al (2012) Ultrasensitive electrochemical immunosensor based on Au nanoparticles dotted carbon nanotube-graphene composite and functionalized mesoporous materials. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2011.11.054
Yan M, Zang D, Ge S et al (2012) A disposable electrochemical immunosensor based on carbon screen-printed electrodes for the detection of prostate specific antigen. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2012.06.019
Mao K, Wu D, Li Y et al (2012) Label-free electrochemical immunosensor based on graphene/methylene blue nanocomposite. Anal Biochem. https://doi.org/10.1016/j.ab.2011.12.047
Kim DJ, Sohn IY, Jung JH et al (2013) Reduced graphene oxide field-effect transistor for label-free femtomolar protein detection. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2012.09.040
Jang HD, Kim SK, Chang H, Choi JW (2015) 3D label-free prostate specific antigen (PSA) immunosensor based on graphene-gold composites. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2014.08.008
Wu D, Liu Y, Wang Y et al (2016) Label-free electrochemiluminescent immunosensor for detection of prostate specific antigen based on aminated graphene quantum dots and carboxyl graphene quantum dots. Sci Rep. https://doi.org/10.1038/srep20511
Assari P, Rafati AA, Feizollahi A, Asadpour Joghani R (2019) An electrochemical immunosensor for the prostate specific antigen based on the use of reduced graphene oxide decorated with gold nanoparticles. Microchim Acta. https://doi.org/10.1007/s00604-019-3565-8
Cho EJ, Lee J-W, Ellington AD (2009) Applications of aptamers as sensors. Annu Rev Anal Chem. https://doi.org/10.1146/annurev.anchem.1.031207.112851
Kaur H (2018) Recent developments in cell-SELEX technology for aptamer selection. Biochim. Biophys. Acta - Gen. Subj.
Fang BY, Wang CY, Li C et al (2017) Amplified using DNase I and aptamer/graphene oxide for sensing prostate specific antigen in human serum. Sensors Actuators B Chem. https://doi.org/10.1016/j.snb.2017.01.045
Wei B, Mao K, Liu N et al (2018) Graphene nanocomposites modified electrochemical aptamer sensor for rapid and highly sensitive detection of prostate specific antigen. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2018.08.067
Maehashi K, Katsura T, Kerman K et al (2007) Label-free protein biosensor based on aptamer-modified carbon nanotube field-effect transistors. Anal Chem. https://doi.org/10.1021/ac060830g
Yun YH, Bange A, Heineman WR et al (2007) A nanotube array immunosensor for direct electrochemical detection of antigen-antibody binding. Sensors Actuators B Chem. https://doi.org/10.1016/j.snb.2006.08.014
Yu X, Kim SN, Papadimitrakopoulos F, Rusling JF (2005) Protein immunosensor using single-wall carbon nanotube forests with electrochemical detection of enzyme labels. Mol Biosyst. https://doi.org/10.1039/b502124c
Yu X, Munge B, Patel V et al (2006) Carbon nanotube amplification strategies for highly sensitive immunodetection of cancer biomarkers. J Am Chem Soc. https://doi.org/10.1021/ja062117e
Malhotra R, Papadimitrakopoulos F, Rusling JF (2010) Sequential layer analysis of protein immunosensors based on single wall carbon nanotube forests. Langmuir. https://doi.org/10.1021/la102306z
Salimi A, Kavosi B, Fathi F, Hallaj R (2013) Highly sensitive immunosensing of prostate-specific antigen based on ionic liquid-carbon nanotubes modified electrode: application as cancer biomarker for prostate biopsies. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2012.10.053
Kavosi B, Salimi A, Hallaj R, Amani K (2014) A highly sensitive prostate-specific antigen immunosensor based on gold nanoparticles/PAMAM dendrimer loaded on MWCNTS/chitosan/ionic liquid nanocomposite. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2013.08.012
Quintero-Jaime AF, Berenguer-Murcia Á, Cazorla-Amorós D, Morallón E (2019) Carbon nanotubes modified with Au for electrochemical detection of prostate specific antigen: Effect of au nanoparticle size distribution. Front Chem. https://doi.org/10.3389/fchem.2019.00147
Farzin L, Sadjadi S, Shamsipur M, Sheibani S (2019) An immunosensing device based on inhibition of mediator’s faradaic process for early diagnosis of prostate cancer using bifunctional nanoplatform reinforced by carbon nanotube. J Pharm Biomed Anal. https://doi.org/10.1016/j.jpba.2019.05.008
Wang J (2005) Nanomaterial-based electrochemical biosensors. Analyst
Chang H, Zhang H, Lv J et al (2016) Pt NPs and DNAzyme functionalized polymer nanospheres as triple signal amplification strategy for highly sensitive electrochemical immunosensor of tumour marker. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2016.06.048
Ferreira AAP, Fugivara CS, Barrozo S et al (2009) Electrochemical and spectroscopic characterization of screen-printed gold-based electrodes modified with self-assembled monolayers and Tc85 protein. J Electroanal Chem. https://doi.org/10.1016/j.jelechem.2009.07.018
Han J, Zhuo Y, Chai Y et al (2012) Simultaneous electrochemical detection of multiple tumor markers based on dual catalysis amplification of multi-functionalized onion-like mesoporous graphene sheets. Anal Chim Acta. https://doi.org/10.1016/j.aca.2012.08.018
Feng J, Li Y, Li M et al (2017) A novel sandwich-type electrochemical immunosensor for PSA detection based on PtCu bimetallic hybrid (2D/2D) rGO/g-C3N4. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2016.12.070
Shahdost-fard F, Roushani M (2017) Designing an ultra-sensitive aptasensor based on an AgNPs/thiol-GQD nanocomposite for TNT detection at femtomolar levels using the electrochemical oxidation of Rutin as a redox probe. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2016.09.048
Cho KY, Seo HY, Yeom YS et al (2016) Stable 2D-structured supports incorporating ionic block copolymer-wrapped carbon nanotubes with graphene oxide toward compact decoration of metal nanoparticles and high-performance nano-catalysis. Carbon N Y. https://doi.org/10.1016/j.carbon.2016.04.049
Thunkhamrak C, Chuntib P, Ounnunkad K et al (2020) Highly sensitive voltammetric immunosensor for the detection of prostate specific antigen based on silver nanoprobe assisted graphene oxide modified screen printed carbon electrode. Talanta. https://doi.org/10.1016/j.talanta.2019.120389
Zhao L, Ma Z (2017) New immunoprobes based on bovine serum albumin-stabilized copper nanoclusters with triple signal amplification for ultrasensitive electrochemical immunosensing for tumor marker. Sensors Actuators, B Chem. https://doi.org/10.1016/j.snb.2016.11.012
Liu X, Yue T, Qi K et al (2020) Porous graphene based electrochemical immunosensor using Cu3(BTC)2 metal-organic framework as nonenzymatic label. Talanta. https://doi.org/10.1016/j.talanta.2020.121042
Kuila T, Bose S, Khanra P, et al (2011) Recent advances in graphene-based biosensors. Biosens. Bioelectron.
Fan D, Li N, Ma H et al (2016) Electrochemical immunosensor for detection of prostate specific antigen based on an acid cleavable linker into MSN-based controlled release system. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2016.05.063
Argoubi W, Sánchez A, Parrado C et al (2018) Label-free electrochemical aptasensing platform based on mesoporous silica thin film for the detection of prostate specific antigen. Sensors Actuators, B Chem. https://doi.org/10.1016/j.snb.2017.08.045
Kavosi B, Salimi A, Hallaj R, Moradi F (2015) Ultrasensitive electrochemical immunosensor for PSA biomarker detection in prostate cancer cells using gold nanoparticles/PAMAM dendrimer loaded with enzyme linked aptamer as integrated triple signal amplification strategy. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2015.07.064
Prensner JR, Rubin MA, Wei JT, Chinnaiyan AM (2012) Beyond PSA: the next generation of prostate cancer biomarkers. Sci. Transl. Med.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Arshid Numan and Sima Singh equally contributed
Highlights
• PSA is a prostate cancer-specific protein biomarker.
• This review mainly focuses on recently developed optical and electrochemical sensors for PSA detection.
• This review discusses the important roles played by nanomaterials in the frequent monitoring of PSA levels at various stages of cancer.
Rights and permissions
About this article
Cite this article
Numan, A., Singh, S., Zhan, Y. et al. Advanced nanoengineered—customized point-of-care tools for prostate-specific antigen. Microchim Acta 189, 27 (2022). https://doi.org/10.1007/s00604-021-05127-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-05127-y