Abstract
A novel biosensing interface for tumor markers was designed based on the atom transfer radical polymerization (ATRP) of poly(isopropenylphenol) (PPPL) in situ initiated by the fixing of p-chloromethyl benzoic acid on the surface of amino-modified electrodes. It was found that the electrochemical activity of PPPL itself can provide sufficient signals for these biosensors, which can avoid signal leakage and streamline the interface modification process. Cu(II) ions absorbed on the carbon spheres and then were released via acid stimulation to act as a catalyst to participate in the interface polymerization with ATRP. As the concentration of targets increased, more Cu(II) ions were released, and the electrochemical signal of polymers was enhanced. Therefore, the sensitive detection of carbohydrate antigen 19–9 (CA19-9) as a model target was achieved, with an ultralow limit of detection of 39 µU mL−1 and wide detection range from 100 µU mL−1 to 100 U mL−1 under optimal conditions. Furthermore, this method achieved satisfying performance in human blood serum with good inter-assay precision (RSD < 6%) and satisfactory recovery of ~ 99–105%. According to the results, this work is of great significance for constructing biosensor interfaces via in situ polymerization.
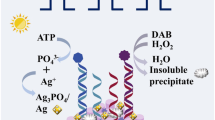
Graphical abstract
A novel biosensing interface for tumor marker was designed based on atom transfer radical polymerization (ATRP), which poly(isopropenylphenol) with electrochemical signal was fabricated in situ on electrode.








Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34
Ferlay J, Colombet M, Soerjomataram I et al (2019) Estimating the global cancer incidence and mortality in 2018: globocan sources and methods. Int J Cancer 144:1941–1953
Karley D, Gupta D, Tiwari A (2011) Biomarker for cancer: a great promise for future. World J Oncol 2:151–157
Rabinowits G, Gerçel-Taylor C, Day JM et al (2009) Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer 10:42–46
Del Villano BC, Brennan S, Brock P et al (1983) Radloimmunometric assay for a monoclonal antibody-defined tumor marker, CA 19–9. Clin Chem 29:549–552
Kaur S, Smith LM, Patel A et al (2017) A combination of MUC5AC and CA19-9 improves the diagnosis of pancreatic cancer: a multicenter study. Am J Gastroenterol 112:172–183
Lequin RM (2005) Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA). Clin Chem 51:2415–2418
Heidari MH, Porghasem M, Mirzaei N et al (2014) The effect of high level natural ionizing radiation on expression of PSA, CA19-9 and CEA tumor markers in blood serum of inhabitants of Ramsar. Iran J Environ Radioact 128:64–67
Babamiri B, Hallaj R, Salimi A et al (2017) Potential-resolved electrochemiluminescence immunoassay for simultaneous determination of CEA and AFP tumor markers using dendritic nanoclusters and Fe3O4@SiO2 nanoparticles. Microchim Acta 184:3613–3623
Chen D, Zhang M, Zhou F et al (2019) Ultrasensitive electroluminescence biosensor for a breast cancer marker microRNA based on target cyclic regeneration and multi-labeled magnetized nanoparticles. Microchim Acta 186:628
Chen H, Luo D, Shang B et al (2020) Immunoassay-type biosensor based on magnetic nanoparticle capture and the fluorescence signal formed by horseradish peroxidase catalysis for tumor-related exosome determination. Microchim Acta 187:282
Wang J, Liu H, Huang X, Ren J (2015) Homogeneous immunoassay for the cancer marker alpha-fetoprotein using single wavelength excitation fluorescence cross-correlation spectroscopy and CdSe/ZnS quantum dots and fluorescent dyes as labels. Microchim Acta 183:749–755
Olmsted IR, Hassanein M, Kussrow A et al (2014) Toward rapid, high-sensitivity, volume-constrained biomarker quantification and validation using backscattering interferometry. Anal Chem 86:7566–7574
Lu N, Gao A, Dai P et al (2015) Ultrasensitive detection of dual cancer biomarkers with integrated CMOS-compatible nanowire arrays. Anal Chem 87:11203–11208
Ibáñez-Redín G, Materon EM, Furuta RHM et al (2020) Screen-printed electrodes modified with carbon black and polyelectrolyte films for determination of cancer marker carbohydrate antigen 19–9. Microchim Acta 187:417
Gao Y, Huo W, Zhang L et al (2019) Multiplex measurement of twelve tumor markers using a GMR multi-biomarker immunoassay biosensor. Biosens Bioelectron 123:204–210
Putnin T, Ngamaroonchote A, Wiriyakun N et al (2019) Dually functional polyethylenimine-coated gold nanoparticles: a versatile material for electrode modification and highly sensitive simultaneous determination of four tumor markers. Microchim Acta 186:305
Guo K, Wustoni S, Koklu A et al (2021) Rapid single-molecule detection of COVID-19 and MERS antigens via nanobody-functionalized organic electrochemical transistors. Nat Biomed Eng. https://doi.org/10.1038/s41551-021-00734-9
Zhang DS, Li WX, Wang HQ, Ma ZF (2018) A novel immunoprobe composed of reduced graphene oxide-hemin-thionin-Au nanohybrid for ultrasensitive detection of tumor marker. Sens Actuators B: Chem 258:141–147
Shen WJ, Zhuo Y, Chai YQ et al (2015) Enzyme-free electrochemical immunosensor based on host-guest nanonets catalyzing amplification for procalcitonin detection. ACS Appl Mater Interfaces 7:4127–4134
Chen Y, Wang AJ, Yuan PX et al (2019) Three dimensional sea-urchin-like PdAuCu nanocrystals/ferrocene-grafted-polylysine as an efficient probe to amplify the electrochemical signals for ultrasensitive immunoassay of carcinoembryonic antigen. Biosens Bioelectron 132:294–301
Huang ZJ, Jiang ZQ, Zhao CF et al (2017) Simple and effective label-free electrochemical immunoassay for carbohydrate antigen 19–9 based on polythionine-Au composites as enhanced sensing signals for detecting different clinical samples. Int J Nanomedicine 12:3049–3058
Meng XZ, Xu Y, Zhang NN et al (2021) Ferric hydroxide nanocage triggered Fenton-like reaction to improve amperometric immunosensor. Sens Actuators B: Chem 338:129840
Cai XH, Weng SH, Guo RB et al (2016) Ratiometric electrochemical immunoassay based on internal reference value for reproducible and sensitive detection of tumor marker. Biosens Bioelectron 81:173–180
Li LD, Zhao HT, Chen ZB et al (2011) Aptamer biosensor for label-free square-wave voltammetry detection of angiogenin. Biosens Bioelectron 30:261–266
Deng CY, Qu FL, Sun HY, Yang M et al (2011) Sensitive electrochemical immunosensor based on enlarged and surface charged gold nanoparticles mediated electron transfer. Sens Actuators B: Chem 160:471–474
Kim S, Sikes HD (2020) Radical polymerization reactions for amplified biodetection signals. Polym Chem 11:1424–1444
Ito H, Willson CG, Frechet JMJ (1983) Synthesis of poly(p-hydroxy-a-methylstyrene) by cationic polymerization and chemical modification. Macromolecules 16:510–517
Choi HY, Lee TJ, Yang GW et al (2017) Generation of integration-free induced neurons using graphene oxide-polyethylenimine. Small 13:1601993
Zheng Y, Ma ZF (2019) Multifunctionalized ZIFs nanoprobe-initiated tandem reaction for signal amplified electrochemical immunoassay of carbohydrate antigen 24–2. Biosens Bioelectron 129:42–49
Choma J, Jamiola D, Augustynek K et al (2012) Carbon-gold core-shell structures: formation of shells consisting of gold nanoparticles. Chem Commun (Camb) 48:3972–3974
Qian H, He L (2009) Detection of protein binding using activator generated by electron transfer for atom transfer radical polymerization. Anal Chem 81:9824–9827
Qian H, He L (2009) Surface-initiated activators generated by electron transfer for atom transfer radical polymerization in detection of DNA point mutation. Anal Chem 81:4536–4542
Zhao HW, Yang GP, Hill AJ et al (2021) One-step ion-exchange from Na-SSZ-13 to Cu-SSZ-13 for NH3-SCR by adjusting the pH value of Cu-exchange solution: The effect of H+ ions on activity and hydrothermal stability. Microporous Mesoporous Mater 324:111271
Loock HP, Wentzell PD (2012) Detection limits of chemical sensors: applications and misapplications. Sens Actuators B: Chem 173:157–163
Montville D, Voigtman E (2003) Statistical properties of limit of detection test statistics. Talanta 59:461–476
Jiao YC, Du C, Zong LJ et al (2020) 3D vertical-flow paper-based device for simultaneous detection of multiple cancer biomarkers by fluorescent immunoassay. Sens Actuators B: Chem 306:127239
Gan N, Xie LS, Zhang K et al (2018) An endonuclease-linked multiplex immunoassay for tumor markers detection based on microfluidic chip electrophoresis for DNA analysis. Sens Actuators B: Chem 272:526–533
Chu W, Chen Y, Liu W et al (2017) Paper-based chemiluminescence immunodevice with temporal controls of reagent transport technique. Sens Actuators B: Chem 250:324–332
Kalyani T, Sangili A, Nanda A et al (2021) Bio-nanocomposite based highly sensitive and label-free electrochemical immunosensor for endometriosis diagnostics application. Bioelectrochemistry 139:107740
Staden RS, Gheorghe SS, Ilie-Mihai RM et al (2021) Disposable stochastic sensor based on deposition of a nanolayer of silver on silk for molecular recognition of specific biomarkers. J Electrochem Soc 168:037515
Funding
This research was financed by grants from Joint project of Beijing Municipal Education Commission and Beijing Natural Science Foundation (KZ202110028042), National Natural Science Foundation of China (22172104, 21673143), and Capacity Building for Sci-Tech Innovation-Fundamental Scientific Research Funds (20530290087, 20530290055).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, F., Xu, Y., Han, H. et al. In situ growth of electroactive polymers via ATRP to construct a biosensing interface for tumor marker. Microchim Acta 188, 389 (2021). https://doi.org/10.1007/s00604-021-05048-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-05048-w