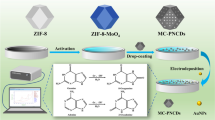
Abstract
An efficient electrochemical biosensor has been developed for the simultaneous evaluation of DNA bases using AgNPs-embedded covalent organic framework (COF). The COF (p-Phenylenediamine and terephthalaldehyde) was synthesized by reflux (DMF; 150 °C; 12 h) and the nanoparticles were embedded from the aqueous solutions of AgNO3 and NaBH4. The nanocomposite-modified COF was confirmed by spectral, microscopic, and electrochemical techniques. The nanocomposite material was deposited on a glassy carbon electrode (GCE) and the redox behavior of AgNPs was confirmed by cyclic voltammetry. The electrocatalytic activities of DNA bases were analyzed by differential pulse voltammetry (DPV) in a physiological environment (PBS; pH = 7.0) based on simple and easy-to-use electrocatalyst. The AgNPs-COF/GCE showed well-defined anodic peak currents for the bases guanine (+ 0.63 V vs. Ag/AgCl), adenine (+ 0.89 V vs. Ag/AgCl), thymine (+ 1.10 V vs. Ag/AgCl), and cytosine (+ 1.26 V vs. Ag/AgCl) in a mixture as well as individuals with respect to the conventional, COF, and AgNPs/GCEs. The AgNPs-COF/GCE showed linear concentration range of DNA bases from 0.2–1000 µM (guanine; (G)), 0.1–500 µM (adenine (A)), 0.25–250 µM (thymine (T)) and 0.15–500 µM (cytosine (C)) and LOD of 0.043, 0.056, 0.062, and 0.051 µM (S/N = 3), respectively. The developed sensor showed reasonable selectivity, reproducibility (RSD = 1.53 ± 0.04%–2.58 ± 0.02% (n = 3)), and stability (RSD = 1.22 ± 0.06%–2.15 ± 0.04%; n = 3) over 5 days of storage) for DNA bases. Finally, AgNPs-COF/GCE was used for the determination of DNA bases in human blood serum, urine and saliva samples with good recoveries (98.60–99.11%, 97.80–99.21%, and 98.69–99.74%, respectively).
Graphical abstract









Similar content being viewed by others
References
Watson JD, Crick FHC (1953) Molecular structure of nucleic acids: a structure for deoxyribose nucleic acid. Nature 171:737–738
Lavanya N, Claude JN, Sekar C (2018) Electrochemical determination of purine and pyrimidine bases using copper doped cerium oxide nanoparticles. J Colloid Interface Sci 530:202–211
Yari A, Saidikhah M (2016) Trithiane silver-nanoparticles-decorated polyaniline nanofibers as sensing element for electrochemical determination of Adenine and Guanine in DNA. J Electroanal Chem 783:288–294
Li T, Li B, Dong S (2007) Aptamer-based label-free method for hemin recognition and DNA assay by capillary electrophoresis with chemiluminescence detection. Anal Bioanal Chem 389:887–893
Qian Y, Fan T, Yao Y, Shi X, Liao X, Zhou F, Gao F (2018) Label-free and Raman dyes-free surface-enhanced Raman spectroscopy for detection of DNA. Sens Actuators B Chem 254:483–489
Doshi R, Day PJR, Carampin P, Blanch E, Stratford IJ, Tirelli N (2010) Spectrophotometric analysis of nucleic acids: oxygenation-dependant hyperchromism of DNA. Anal Bioanal Chem 396:2331–2339
Fujimura YK, Komatsu Y, Kuraishi H, Kaneko T (1984) Estimation of DNA base composition by high performance liquid chromatography of its nuclease pi hydrolysate. Agric BioI Chem 48:3169–3172
Rehman A, Jenner A, Halliwell B (2000) Gas chromatography-mass spectrometry analysis of DNA: optimization of protocols for isolation and analysis of DNA from human blood. Methods Enzymol 319:401–417
Yeh CF, Jiang SJ (2002) Determination of monophosphate nucleotides by capillary electrophoresis inductively coupled plasma mass spectrometry. Analyst 127:1324–1327
Brooks SC, Richter MM (2002) Determination of DNA bases using electrochemistry: a discovery-based experiment. Chem Educat 7:284–287
Sadeghi M, Jahanshahi M, Javadian H (2020) Highly sensitive biosensor for detection of DNA nucleobases: enhanced electrochemical sensing based on polyaniline/single-layer MoS2 nanosheets nanocomposite modified carbon paste electrode. Microchem J. 152:104315
Lei P, Zhou Y, Zhu R, Liu Y, Dong C, Shuang S (2020) Novel strategy of electrochemical analysis of DNA bases with enhanced performance based on copper nickel nanosphere decorated N, B doped reduced graphene oxide. Biosens Bioelectron 147:111735
Wang S, Ferrag C, Noroozifar M, Kerman K (2020) Simultaneous determination of four DNA bases at graphene oxide/multi-walled carbon nanotube nanocomposite-modified electrode. Micromachines 11:294
Wang X, Zhang J, Wei Y, Xing T, Cao T, Wu S, Zhu F (2020) A copper-based metal–organic framework/graphene nanocomposite for the sensitive and stable electrochemical detection of DNA bases. Analyst 145:1933–1942
Deng C, Xia Y, Xiao C, Nie Z, Yang M, Si S (2012) Electrochemical oxidation of purine and pyrimidine bases based on the boron-doped nanotubes modified electrode. Biosens and Bioelectron 31:469–474
Wang L, Yu F, Wang F, Chen Z (2016) Electrochemical detection of DNA methylation using a glassy carbon electrode modified with a composite made from carbon nanotubes and β-cyclodextrin. J Solid State Electrochem 20:1263–1270
Wu K, Fei J, Bai W, Hu S (2003) Direct electrochemistry of DNA, guanine and adenine at a nanostructured film-modified electrode. Anal Bioanal Chem 376:205–209
Luo X, Morrin A, Killard AJ, Smyth MR (2006) Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 18:319–326
Wang J (2012) Electrochemical biosensing based on noble metal nanoparticles. Microchim Acta 177:245–270
Mavaei M, Chahardoli A, Shokoohinia Y, Khoshroo A, Fattahi A (2020) One-step synthesized silver nanoparticles using isoimperatorin: evaluation of photocatalytic, and electrochemical activities. Sci Rep 10:1762
Lu L, Hu X, Zhu Z, Li D, Tian S, Chen Z (2020) Review-Electrochemical sensors and biosensors modified with binary nanocomposite for food safety. J Electrochem Soc 167:037512
Manojkumar K, Sivaramakrishna A, Vijayakrishna K (2016) A short review on stable metal nanoparticles using ionic liquids, supported ionic liquids, and poly(ionic liquids). J Nanopart Res 18:103
Pan M, Yang J, Liu K, Yin Z, Ma T, Liu S, Xu L, Wang S (2020) Noble metal nanostructured materials for chemical and biosensing systems. Nanomater 10:209
Xie F, Yang M, Jiang M, Huang XJ, Liu WQ, Xie PH (2019) Carbon-based nanomaterials A promising electrochemical sensor toward persistent toxic substance. Trends Anal Chem 119:115624
Wu S, He Q, Tan C, Wang Y, Zhang H (2013) Graphene-based electrochemical sensors. Small 9:1160–1172
Liu CS, Li J, Pang H (2020) Metal-organic framework-based materials as an emerging platform for advanced electrochemical sensing. Coord Chem Rev 410:213222
Zhang J, Tan Y, Song WJ (2020) Zeolitic imidazolate frameworks for use in electrochemical and optical chemical sensing and biosensing: a review. Microchim Acta 187:234
Geng K, He T, Liu R, Dalapati S, Tan KT, Li Z, Tao S, Gong Y, Jiang Q, Jiang D (2020) Covalent organic frameworks: design, synthesis, and functions. Chem Rev 120:8814–8933
Lohse MS, Bein T (2018) Covalent organic frameworks: structures, synthesis, and applications. Adv Funct Mater 28:1705553
Gomes R, Bhanja P, Bhaumik A (2015) A triazine-based covalent organic polymer for efficient CO2 adsorption. Chem Commun 51:10050–10053
Sayyah SM, Khaliel AB, Aboud AA, Mohamed SM (2014) Chemical polymerization kinetics of poly-o-phenylenediamine and characterization of the obtained polymer in aqueous hydrochloric acid solution using K2Cr2O7 as oxidizing agent. Int J Polym Sci 16:520910
Qu F, Yan H, Li K, You J (2020) Han W (2020) A covalent organic framework–MnO2 nanosheet system for determination of glutathione. J Mater Sci 55:10022–10034
Pareek K, Rohana R, Cheng H (2015) Polymeric organo–magnesium complex for room temperature hydrogen physisorption. RSC Adv 5:10886–10891
Arul P, John SA (2017) Silver nanoparticles built-in zinc metal organic framework modified electrode for the selective non-enzymatic determination of H2O2. Electrochimi Acta 235:680–689
Arul P, Narayanamoorthi E, John SA (2020) Covalent organic framework film as an effective electrocatalyst for the simultaneous determination of dihydroxybenzene isomers in water samples. Sens Actuators B Chem 313:128033
Ouyang X, Luo L, Ding Y, Liu B, Xu D (2014) Simultaneous determination of purine and pyrimidine bases in DNA using poly(3,4-ethylenedioxythiophene)/graphene composite film. J Electroanal Chem 735:51–56
Xu Q, Liu X, Li H, Yin L, Hu X (2013) Electrochemical determination of purine and pyrimidine DNA bases based on the recognition properties of azocalix[4]arene. Biosens Bioelectron 42:355–361
Ren S, Wang H, Zhang H, Yu L, Li M, Li M (2015) Direct electrocatalytic and simultaneous determination of purine and pyrimidine DNA bases using novel mesoporous carbon fibers as electrocatalyst. J Electroanal Chem 750:65–73
Funding
The authors are grateful for the financial support from the Ministry of Science and Technology, Taiwan (MOST-107–2113-M-027–006 and MOST-108–2113-M-027–001). P. Arul would like to express gratitude to the National Taipei University of Technology for the Post-doctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arul, P., Huang, ST., Gowthaman, N.S.K. et al. Simultaneous electrochemical determination of DNA nucleobases using AgNPs embedded covalent organic framework. Microchim Acta 188, 358 (2021). https://doi.org/10.1007/s00604-021-05021-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-05021-7