Abstract
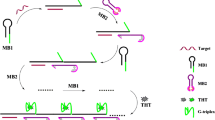
A novel strategy for microRNAs (miRNAs) detection has been developed utilizing duplex-specific nuclease-assisted signal amplification (DSNSA) and guanine-rich DNA-enhanced fluorescence of DNA-templated silver nanoclusters (AgNCs). The combination between target miRNA, DSNSA, and AgNCs is achieved by the unique design of DNA sequences. Target miRNA opens the hairpin structure of the Hairpin DNA probe (HP) by hybridizing with the HP and initiates the duplex-specific nuclease-assisted signal amplification (DSNSA) reaction. The DSNSA reaction generates the release of the guanine-rich DNA sequence, which can turn on the fluorescence of the dark AgNCs by hybridizing with the DNA template of the dark AgNCs. The fluorescence intensity of AgNCs corresponds to the dosage of the target miRNA. This is measured at 630 nm by exciting at 560 nm. The constructed method exhibits a low detection limit (~8.3 fmol), a great dynamic range of more than three orders of magnitude, and excellent selectivity. Moreover, it has a good performance for miR-21 detection in complex biological samples.
Graphical abstract

A novel strategy for microRNAs (miRNAs) detection has been developed utilizing duplex-specific nuclease-assisted signal amplification (DSNSA) and guanine-rich DNA-enhanced fluorescence of DNA-templated silver nanoclusters (AgNCs).





Similar content being viewed by others
References
Petty JT, Zheng J, Hud NV, Dickson RM (2004)DNA-templated Ag nanocluster formation. J Am Chem Soc 126:5207–5212. https://doi.org/10.1021/ja031931o
Su YT, Lan GY, Chen WY, Chang HT (2010) Detection of copper ions through recovery of the fluorescence of DNA-templatedcopper/silver nanoclusters in the presence of mercaptopropionic acid. Anal Chem 82:8566–8572. https://doi.org/10.1021/ac101659d
Guo W, Yuan J, Wang E (2009)Oligonucleotide-stabilized Ag nanoclusters as novel fluorescence probes for the highly selective and sensitive detection of the Hg2+ ion. Chem Commun 23:3395–3397. https://doi.org/10.1039/b821518a
Shen C, Xia X, Hu S, Yang M, Wang J (2015) Silver nanoclusters-based fluorescence assay of protein kinase activity and inhibition. Anal Chem 87:693–698. https://doi.org/10.1021/ac503492k
Han B, Wang E (2011)Oligonucleotide-stabilized fluorescent silver nanoclusters for sensitive detection of biothiols in biological fluids. Biosens Bioelectron 26:2585–2589. https://doi.org/10.1016/j.bios.2010.11.011
New SY, Lee ST, Su XD (2016)DNA-templated silver nanoclusters: structural correlation and fluorescence modulation. Nanoscale 8:17729–17746. https://doi.org/10.1039/c6nr05872h
Huang Z, Pu F, Lin Y, Ren J, Qu X (2011) Modulating DNA-templated silver nanoclusters for fluorescence turn-on detection of thiol compounds. Chem Commun 47:3487–3489. https://doi.org/10.1039/c0cc05651k
Yeh HC, Sharma J, Han JJ, Martinez JS, Werner JH (2010) A DNA-silver nanocluster probe that fluoresces upon hybridization. Nano Lett 10:3106–3110. https://doi.org/10.1021/nl101773c
Li J, Zhong X, Zhang H, Le XC, Zhu JJ (2012)Binding-induced fluorescence turn-on assay using aptamer-functionalized silver nanocluster DNA probes. Anal Chem 84:5170–5174. https://doi.org/10.1021/ac3006268
Yeh HC, Sharma J, Shih IM, Vu DM, Martinez JS, Werner JH (2012) A fluorescence light-up Ag nanocluster probe that discriminates single-nucleotide variants by emission color. J Am Chem Soc 134:11550–11558. https://doi.org/10.1021/ja3024737
Ye T, Chen J, Liu Y, Ji X, Zhou G, He Z (2014) Periodic fluorescent silver clusters assembled by rolling circle amplification and their sensor application. ACS Appl Mater Interfaces 6:16091–16096. https://doi.org/10.1021/am504035a
He Y, Jiao B (2017) Determination of the activity of alkaline phosphatase based on the use of ssDNA-templated fluorescent silver nanoclusters and on enzyme-triggered silver reduction. Microchim Acta 184:4167–4173. https://doi.org/10.1007/s00604-017-2459-x
He Y, Jiao B (2016) Detection of biotin-streptavidin interactions via terminal protection of small molecule linked DNA and the formation of fluorescent DNA-templated silver nanoclusters. Microchim Acta 183:3183–3189. https://doi.org/10.1007/s00604-016-1968-3
Mellis D, Caporali A (2018)MicroRNA-based therapeutics in cardiovascular disease: screening and delivery to the target. Biochem Soc Trans 46:11–21. https://doi.org/10.1042/BST20170037
Beermann J, Piccoli MT, Viereck J, Thum T (2016)Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev 96:1297–1325. https://doi.org/10.1152/physrev.00041.2015
Shah P, Bristow MR, Port JD (2017) MicroRNAs in heart failure, cardiac transplantation, and myocardial recovery: biomarkers with therapeutic potential. Current Heart Failure Reports 14:454–464. https://doi.org/10.1007/s11897-017-0362-8
Snowhite IV, Allende G, Sosenko J, Pastori RL, Cayetano SM, Pugliese A (2017) Association of serum microRNAs with islet autoimmunity, disease progression and metabolic impairment in relatives at risk of type 1 diabetes. Diabetologia 60:1409–1422. https://doi.org/10.1007/s00125-017-4294-3
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR (2005) MicroRNA expression profiles classify human cancers. Nature 435:834–838. https://doi.org/10.1038/nature03702
Cissell KA, Shrestha S, Deo SK (2007) MicroRNA detection: challenges for the analytical chemist. Anal Chem 79:4754–4761. https://doi.org/10.1021/ac0719305
He SL, Green R (2013) Northern blotting. Methods Enzymol 530:75–87. https://doi.org/10.1016/B978-0-12-420037-1.00003-8
Várallyay É, Burgyán J, Havelda Z (2008) MicroRNA detection by northern blotting using locked nucleic acid probes. Nat Protoc 3:190–196. https://doi.org/10.1038/nprot.2007.528
Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM (2002) Frequent deletions and down-regulation of microRNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci 99:15524–15529. https://doi.org/10.1073/pnas.242606799
Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS (2003) A microRNA array reveals extensive regulation of microRNAs during brain development. RNA 9: 1274-1281. 10.1261rna.5980303
Thomson JM, Parker J, Perou CM, Hammond SM (2004) A custom microarray platform for analysis of microRNA gene expression. Nat Methods 1:47–53. https://doi.org/10.1038/NMETH704
Raymond CK, Roberts BS, Garrett-Engele P, Lim LP, Johnson JM (2005) Simple, quantitative primer-extension PCR assay for direct monitoring of microRNAs and short-interfering RNAs. RNA 11:1737–1744. https://doi.org/10.1261/rna.2148705
Yan JL, Li ZP, Liu CH, Cheng YQ (2010) Simple and sensitive detection of microRNAs with ligase chain reaction. Chem Commun 46:2432–2434. https://doi.org/10.1039/b923521c
Zhang PB, Zhang JY, Wang CL, Liu CH, Wang H, Li ZP (2014) Highly sensitive and specific multiplexed microRNA quantification using size-coded ligation chain reaction. Anal Chem 86:1076–1082. https://doi.org/10.1021/ac4026384
Jonstrup SP, Koch J, Kjems J (2006) A microRNA detection system based on padlock probes and rolling circle amplification. RNA 12:1747–1752. https://doi.org/10.1261/rna.110706
Cheng YQ, Zhang X, Li ZP, Jiao XX, Wang YC, Zhang YL (2009) Highly sensitive determination of microRNA using target-primed and branched rolling-circle amplification. Angew Chem Int Ed 48:3268–3272. https://doi.org/10.1002/anie.200805665
Zhou C, Huang R, Zhou XM, Xing D (2020) Sensitive and specific microRNA detection by RNA dependent DNA ligation and rolling circle optical signal amplification. Talanta 216:120954. https://doi.org/10.1016/j.talanta.2020.120954
Jia HX, Li ZP, Liu CH, Cheng YQ (2010) Ultrasensitive detection of microRNAs by exponential isothermal amplification. Angew Chem Int Ed 49:5498–5501. https://doi.org/10.1002/anie.201001375
Li CP, Li ZP, Jia HX, Yan JL (2011)One-step ultrasensitive detection of microRNAs with loop-mediated isothermal amplification (LAMP). Chem Commun 47:2595–2597. https://doi.org/10.1039/c0cc03957h
Du WF, Lv MM, Li JJ, Yu RQ, Jiang JH (2016) A ligation-based loop-mediated isothermal amplification (ligation-LAMP) strategy for highly selective microRNA detection. Chem Commun 52:12721–12724. https://doi.org/10.1039/c6cc06160e
Yin BC, Liu YQ, Ye BC (2012) One-step, multiplexed fluorescence detection of microRNAs based on duplex-specific nuclease signal amplification. J Am Chem Soc 134:5064–5067. https://doi.org/10.1021/ja300721s
Hu ZZ, Chen J, Li WY, Wang Y, Li YX, Sang LJ, Li JM, Zhang QF, Ibupoto ZH, Yu C (2015)Label-free fluorescence turn-on detection of microRNA based on duplex-specific nuclease and a perylene probe. Anal Chim Acta 895:89–94. https://doi.org/10.1016/j.aca.2015.08.028
Xu F, Dong H, Cao Y, Lu H, Meng X, Dai W, Zhang X, Al-Ghanim KA, Mahboob S (2016) Ultrasensitive and multiple disease-related microRNA detection based on tetrahedral DNA nanostructures and duplex-specific nuclease-assisted signal amplification. ACS Appl Mater Interfaces 8:33499–33505. https://doi.org/10.1021/acsami.6b12214
Kumarswamy R, Volkmann I, Thum T (2011) Regulation and function of miRNA-21 in health and disease. RNA Biol 8:706–713. https://doi.org/10.4161/rna.8.5.16154
Vosch T, Antoku Y, Hsiang JC, Richards CI, Gonzalez JI, Dickson RM (2007) Strongly emissive individual DNA-encapsulated Ag nanoclusters as single-molecule fluorophores. Proc Natl Acad Sci 104:12616–12621. https://doi.org/10.1073/pnas.0610677104
Heinlein T, Knemeyer JP, Piestert O, Sauer M (2003) Photoinduced electron transfer between fluorescent dyes and guanosine residues in DNA-hairpins. J Phys Chem B 107:7957–7964. https://doi.org/10.1021/jp0348068
Maretti L, Billone PS, Liu Y, Scaiano JC (2009) Facile photochemical synthesis and characterization of highly fluorescent silver nanoparticles. J Am Chem Soc 131:13972–13980. https://doi.org/10.1021/ja900201k
Yang SW, Vosch T (2011) Rapid detection of microRNA by a silver nanocluster DNA probe. Anal Chem 83:6935–6939. https://doi.org/10.1021/ac201903n
Zhang J, Li C, Zhi X, Ramón GA, Liu Y, Zhang C, Pan F, Cui D (2016) Hairpin DNA-templated silver nanoclusters as novel beacons in strand displacement amplification for microRNAs detection. Anal Chem 88:1294–1302. https://doi.org/10.1021/acs.analchem.5b03729
Kim H, Kang S, Park KS, Park HG (2018)Enzyme-free and label-free miRNA detection based on target-triggered catalytic hairpin assembly and fluorescence enhancement of DNA-silver nanoclusters. Sensors Actuators B Chem 260:140–145. https://doi.org/10.1016/j.snb.2017.12.137
Zhang X, Liu S, Song X, Wang H, Wang J, Wang Y, Huang J, Yu J (2019) DNA three-way junction-actuated strand displacement for miRNA detection using a fluorescence light-up Ag nanocluster probe. Analyst 144:3836–3842. https://doi.org/10.1039/c9an00508k
Pan M, Liang M, Sun J, Liu X, Wang F (2018) Lighting up fluorescent silver clusters via target-catalyzed hairpin assembly for amplified biosensing. Langmuir 34:14851–14857. https://doi.org/10.1021/acs.langmuir.8b01576
Han Y, Zhang F, Gong H, Cai C (2019) Multifunctional G-quadruplex-based fluorescence probe coupled with DNA-templated AgNCs for simultaneous detection of multiple DNAs and MicroRNAs. Anal Chim Acta 1053:105–113. https://doi.org/10.1016/j.aca.2018.11.062
Funding
The project is supported by the National Natural Science Foundation of China (21707027), the Natural Science Foundation of Hebei Province (B2016201052), the Natural Science Foundation of Hebei Education Department (QN2017024), and China Postdoctoral Science Foundation (2017M621094).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 631 kb)
Rights and permissions
About this article
Cite this article
Ma, Gm., Huo, Lw., Tong, Yx. et al. Label-free and sensitive MiRNA detection based on turn-on fluorescence of DNA-templated silver nanoclusters coupled with duplex-specific nuclease-assisted signal amplification. Microchim Acta 188, 355 (2021). https://doi.org/10.1007/s00604-021-05001-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-05001-x