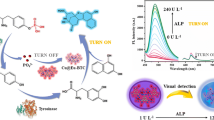
Abstract
A smartphone-based dual-wavelength digital imaging platform containing red (539–695 nm) and blue (389–511 nm) band-pass filters was developed for point-of-care (POC) testing of alkaline phosphatase (ALP) activity. The platform was based on dual-emitting fluorescent nanohybrids (AuNC@NAN), the ratiometric probe, which had a fluorescence “on-off-on-off” response. The probe comprised red-emitting gold nanoclusters (AuNCs) acting as the signal report units and blue-emitting N-(2-aminoethyl-1,8-naphthalimide) (NAN) acting as an internal reference. The different responses of the ratiometric probes resulted in a continuous color-multiplexing change from pink-red to dark-purple upon exposure to ALP. The dual-wavelength digital imaging platform was employed to acquire images of AuNC or NAN fluorescence signals without the influence of background light. Unlike the classical one-time digital imaging mode, the accurate red (R) and blue (B) channel values of the generated images can help to directly judge or eliminate the disturbance from unavoidable interfering factors. The R/B values were successfully employed for determining the ALP activity at a range 2.0 to 35.0 mU·mL−1 with the detection limit of 1.04 mU·mL−1. Such sensing imaging platform is also successful in determining ALP activity in human serum with 94.9–105% recoveries and relative standard deviation in the range 4.2–5.6%.
Graphical abstract

A novel dual-wavelength smartphone-based digital imaging platform was proposed for simultaneous readout of the reporting and internal reference signals from dual-emitting ratiometric fluorescence probes, which allowed us to the accurate, reliable, and highly sensitive assay of ALP activity in complex samples.





Similar content being viewed by others
References
Coleman JE (1992) Structure and mechanism of alkaline phosphatase. Annu Rev Biophys Biomol Struct 21:441–483. https://doi.org/10.1146/annurev.bb.21.060192.002301
Harris H (1990) The human alkaline phosphatases: what we know and what we don’t know. Clin Chim Acta 186:133–150. https://doi.org/10.1016/0009-8981(90)90031-M
McComb RB, Bowers GN Jr, Posen S (2013) Alkaline phosphatase. Springer Science & Business Media, Germany
Colombatto P, Randone A, Civitico G, Gorin JM, Dolci L, Medaina N, Oliveri F, Verme G, Marchiaro G, Pagni R, Karayiannis P, Thomas HC, Hess G, Bonino F, Brunetto MR (1996) Hepatitis G virus RNA in the serum of patients with elevated gamma glutamyl transpeptidase and alkaline phosphatase: a specific liver disease. J Viral Hepat 3:301–306. https://doi.org/10.1111/j.1365-2893.1996.tb00102.x
Couttenye MM, D’Haese PC, Van Hoof VO, Lemoniatou E, Goodman W, Verpooten GA, De Broe ME (1996) Low serum levels of alkaline phosphatase of bone origin: a good marker of adynamic bone disease in haemodialysis patients. Nephrol Dial Transplant 11:1065–1072. https://doi.org/10.1093/ndt/11.6.1065
Goldberg DM, Martin JV, Knight AH (1977) Elevation of serum alkaline phosphatase activity and related enzymes in diabetes mellitus. Clin Biochem 10:8–11. https://doi.org/10.1016/S0009-9120(77)90116-3
Lorente JA, Valenzuela H, Morote J, Gelabert A (1999) Serum bone alkaline phosphatase levels enhance the clinical utility of prostate specific antigen in the staging of newly diagnosed prostate cancer patients. Eur J Nucl Med Mol Imaging 26:625–632. https://doi.org/10.1007/s002590050430
Liu HJ, Li M, Xia YN, Ren XQ (2017) A turn-on fluorescent sensor for selective and sensitive detection of alkaline phosphatase activity with gold nanoclusters based on inner filter effect. ACS Appl Mater Interfaces 9:120–126. https://doi.org/10.1021/acsami.6b11920
Li DX, Qin J, Xu Q, Yan GQ (2018) A room-temperature phosphorescence sensor for the detection of alkaline phosphatase activity based on Mn-doped ZnS quantum dots. Sensors Actuators B Chem 274:78–84. https://doi.org/10.1016/j.snb.2018.07.108
Zhang J, He LF, Zhang X, Wang JP, Yang L, Liu BH, Jiang CL, Zhang ZP (2017) Colorimetric and SERS dual-readout for assaying alkaline phosphatase activity by ascorbic acid induced aggregation of Ag coated Au nanoparticles. Sensors Actuators B Chem 253:126653–126664. https://doi.org/10.1016/j.snb.2017.06.186
Tang ZW, Chen HT, He HL, Ma CB (2019) Assays for alkaline phosphatase activity: progress and prospects. Trac-Trend Anal Chem 113:32–43. https://doi.org/10.1016/j.trac.2019.01.019
Yang JJ, Zheng L, Wang Y, Li W, Zhang JJ, Gu JJ, Fu Y (2016) Guanine-rich DNA-based peroxidase mimetics for colorimetric assays of alkaline phosphatase. Biosens Bioelectron 77:49–556. https://doi.org/10.1016/j.bios.2015.10.003
Kang WJ, Ding YY, Zhou H, Liao QY, Yang X, Yang YG, Jiang JS, Yang MH (2015) Monitoring the activity and inhibition of alkaline phosphatase via quenching and restoration of the fluorescence of carbon dots. Microchim Acta 182:1161–1167. https://doi.org/10.1007/s00604-014-1439-7
Lv ZX, Wang QQ, Yang MH (2021) DNAzyme-Au nanoprobe coupled with graphene-oxide-loaded hybridization chain reaction signal amplification for fluorometric determination of alkaline phosphatase. Microchim Acta 188:7. https://doi.org/10.1007/s00604-020-04681-1
Ma L, Han X, Xia L, Qu FL, Kong RM (2020) A label-free G-quadruplex-based fluorescence assay for sensitive detection of alkaline phosphatase with the assistance of Cu2+. Spectrochim Acta Part A 227:117607–117612. https://doi.org/10.1016/j.saa.2019.117607
Wang YJ, Zeinhom MMA, Yang MM, Sun RR, Wang SF, Smith JN, Timchalk C, Li L, Lin YH, Du D (2017) A 3D-printed, portable, optical-sensing platform for smartphones capable of detecting the herbicide 2,4-dichlorophenoxyacetic acid. Anal Chem 89:9339–9346. https://doi.org/10.1021/acs.analchem.7b02139
Roda A, Michelini E, Zangheri M, Fusco MD, Calabria D, Simoni P (2016) Smartphone-based biosensors: a critical review and perspectives. Trac-Trend Anal Chem 79:317–325. https://doi.org/10.1016/j.trac.2015.10.019
Russell ST, Rica RDL (2018) Policy considerations for mobile biosensors. ACS Sens 3:1059–1068. https://doi.org/10.1021/acssensors.8b00289
Wei QS, Nagi R, Sadeghi K, Feng S, Yan E, Ki SJ, Caire R, Tseng D, Ozcan A (2014) Detection and spatial mapping of mercury contamination in water samples using a smartphone. ACS Nano 8:1121–1129. https://doi.org/10.1021/nn406571t
Gotor R, Ashokkumar P, Hecht M, Keil K, Rurack K (2017) Optical pH sensor covering the range from pH 0-14 compatible with mobile-device readout and based on a set of rationally designed indicator dyes. Anal Chem 89:8437–8444. https://doi.org/10.1021/acs.analchem.7b01903
Rong MC, Deng XZ, Chi ST, Huang LZ, Zhou YB, Shen YN, Chen X (2018) Ratiometric fluorometric determination of the anthrax biomarker 2,6-dipicolinic acid by using europium(III)-doped carbon dots in a test stripe. Microchim Acta 185:201–210. https://doi.org/10.1007/s00604-018-2741-6
Lan MH, Wu SL ZSJ, Wei XF, Fu YZ, Wu JJ, Wang PF, Zhang WJ (2019) Optically tunable fluorescent carbon nanoparticles and their application in fluorometric sensing of copper ions. Nano Res 12:2576–2583. https://doi.org/10.1007/s12274-019-2489-2
Li XC, Zhao SJ, Li BL, Yang K, Lan MH, Zeng LT (2021) Advances and perspectives in carbon dot-based fluorescent probes: mechanism, and application. Coord Chem Rev 431:213686. https://doi.org/10.1016/j.ccr.2020.213686
Zhang J, Qian JJ, Mei QS, Yang L, He LF, Liu SJ, Zhang C, Zhang K (2019) Imaging-based fluorescent sensing platform for quantitative monitoring and visualizing of fluoride ions with dual-emission quantum dots hybrid. Biosens Bioelectron 128:61–67. https://doi.org/10.1016/j.bios.2018.12.044
Hou L, Qin YX, Li JY, Qin SY, Huang YL, Lin TR, Guo LQ, Ye FG, Zhao SL (2019) A ratiometric multicolor fluorescence biosensor for visual detection of alkaline phosphatase activity via a smartphone. Biosens Bioelectron 143:111605–111611. https://doi.org/10.1016/j.bios.2019.111605
You XR, Huang CY, Luo YX, Shi GY, Zhou TS, Deng JJ (2020) A smartphone-based platform for point-of-use determination of alkaline phosphatase as an indicator of water eutrophication. Microchim Acta 187:354–363. https://doi.org/10.1007/s00604-020-04336-1
Liu YQ, Lin XD, Ji XY, Hao Z, Tao ZH (2020) Smartphone-based enzyme-free fluorescence sensing of organophosphate DDVP. Microchim Acta 187:419–426. https://doi.org/10.1007/s00604-020-04384-7
Bueno D, Muñoz R, Marty JL (2016) Fluorescence analyzer based on smartphone camera and wireless for detection of ochratoxin A. Sensors Actuators B Chem 232:462–468. https://doi.org/10.1016/j.snb.2016.03.140
Qian JJ, Cao NN, Zhang J, Hou JJ, Chen Q, Zhang C, Sun YD, Liu SJ, He LF, Zhang K, Zhou HB (2020) Field-portable ratiometric fluorescence imaging of dual-color label-free carbon dots for uranyl ions detection with cellphone-based optical platform. Chin Chem Lett 31:2925–2928. https://doi.org/10.1016/j.cclet.2020.05.004
Mei QS, Jing HR, Li Y, Yisibashaer W, Chen J, Li BN, Zhang Y (2016) Smartphone based visual and quantitative assays on up conversional paper sensor. Biosens Bioelectron 75:427–432. https://doi.org/10.1016/j.bios.2015.08.054
Zhu HJ, Tao Y, Xu HD, Zhang K, Jiang H, Zhang ZP, Wang ZY, Wang SH (2014) Fluorescent nanohybrid of gold nanoclusters and quantum dots for visual determination of lead ions. ACS Appl Mater Interfaces 6:21461–21467. https://doi.org/10.1021/am5064603
Huang XY, Zhou YJ, Liu C, Zhang RL, Zhang LY, Du SH, Liu BH, Han MY, Zhang ZP (2016) A single dual-emissive nanofluorophore test paper for highly sensitive colorimetry-based quantification of blood glucose. Biosens Bioelectron 86:530–535. https://doi.org/10.1016/j.bios.2016.07.021
Sun JY, Mei H, Wang SF, Gao F (2016) Two-photon semiconducting polymer dots with dual-emission for ratiometric fluorescent sensing and bioimaging of tyrosinase activity. Anal Chem 88:372–7377. https://doi.org/10.1021/acs.analchem.6b01929
Lee WI, Shrivastava S, Duy LT, Kim BY, Son YM, Lee NE (2017) A smartphone imaging-based label-free and dual-wavelength fluorescent biosensor with high sensitivity and accuracy. Biosens Bioelectron 94:643–650. https://doi.org/10.1016/j.bios.2017.03.061
Sun J, Yang F, Zhao D, Yang XR (2014) Highly sensitive real-time assay of inorganic pyrophosphatase activity based on the fluorescent gold nanoclusters. Anal Chem 86:7883–7889. https://doi.org/10.1021/ac501814u
Sun J, Yang XR (2015) Gold nanoclusters-Cu2+ ensemble-based fluorescence turn-on and real-time assay for acetylcholinesterase activity and inhibitor screening. Biosens Bioelectron 74:177–182. https://doi.org/10.1016/j.bios.2015.06.013
Jisha VS, Thomas AJ, Ramaiah D (2009) Fluorescence ratiometric selective recognition of Cu2+ ions by dansyl-naphthalimide dyads. J Org Chem 74:6667–6673. https://doi.org/10.1021/jo901164w
Yu H, Chen XF, Yu L, Sun MT, Alamry KA, Asiri AM, Zhang K, Zapien JA, Wang SH (2018) Fluorescent MUA-stabilized Au nanoclusters for sensitive and selective detection of penicillamine. Anal Bioanal Chem 410:2629–2636. https://doi.org/10.1007/s00216-018-0936-7
Sun J, Zhang J, Jin YD (2013) 11-Mercaptoundecanoic acid directed one-pot synthesis of water-soluble fluorescent gold nanoclusters and their use as probes for sensitive and selective detection of Cr3+ and Cr6+. J Mater Chem C 1:138–143. https://doi.org/10.1039/C2TC00021K
Chu YH, Yu XX, Jin X, Wang YT, Zhao DJ, Zhang P, Sun GM, Zhang YH (2019) Purification and characterization of alkaline phosphatase from lactic acid bacteria. RSC Adv 9:354–360. https://doi.org/10.1039/C8RA08921C
Funding
This work is financially supported by the National Natural Science Foundation of China (Nos. 21976002, 22004003, 21675158, and 21507134), and the Natural Science Foundation of Anhui Province (Nos. 1908085MB41 and 1908085QB75).
Author information
Authors and Affiliations
Contributions
Nana Cao and Jinjin Hou carried out the experiments and collected the date. Qihou Chen and Jian Zhang designed and conceived the whole experiment. Cheng Zhang and Yudie Sun worked out the data analysis and interpretation. Jian Zhang wrote and revised this manuscript. Qian Chen and Lifang He revised and edited this manuscript. Kui Zhang oversaw the whole experiment and manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 1503 kb)
Rights and permissions
About this article
Cite this article
Cao, N., Hou, J., Chen, Q. et al. Band-pass filter–assisted ratiometric fluorescent nanoprobe composed of N-(2-aminoethyl-1,8-naphthalimide)-functionalized gold nanoclusters for the determination of alkaline phosphatase using digital image analysis. Microchim Acta 188, 218 (2021). https://doi.org/10.1007/s00604-021-04870-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04870-6