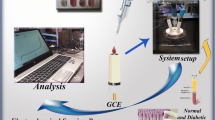
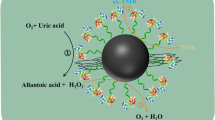
Abstract
A nanozyme based on graphene oxide modified with Fe3O4 NPs, CuO NPs, and cucurbit[6]uril has been successfully fabricated by a simple sonochemical technique. By employing CB[6] as a specific binding pocket and Fe3O4@CuO-GO as a peroxidase mimic, this novel nanozyme (BN I) is equipped with molecular recognition ability and enhanced peroxidase-like activity. On the basis of the inhibition effect of homocysteine (Hcy) towards the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) catalyzed by BN I, a simple colorimetric method is established for the sensitive and selective determination of Hcy. This proposed method displays a good linear response in the range 5–200 μM with a detection limit of 1.8 μM. In the practical assay of human plasma samples, the relative standard deviations (RSD) are lower than 11% and the recoveries are between 98.0 and 104.9%. In the assay of human urine samples, the RSD are below 9.0% and the recoveries range from 94.0 to 103.5%. The colorimetric method presented offers a convenient and accurate way for the determination of biomarkers in point-of-care testing (POCT).
Graphical abstract







Similar content being viewed by others
References
Ye H, Yang K, Tao J, Liu Y, Zhang Q, Habibi S, Nie Z, Xia X (2017) An enzyme-free signal amplification technique for ultrasensitive colorimetric assay of disease biomarkers. ACS Nano 11:2052–2059. https://doi.org/10.1021/acsnano.6b08232
Alam SF, Kumar S, Ganguly P (2019) Measurement of homocysteine: a historical perspective. J Clin Biochem Nutr 65:171–177. https://doi.org/10.3164/jcbn.19-49
Espina JG, Montes-Bayón M, Sanz-Medel A (2015) Determination of reduced homocysteine in human serum by elemental labelling and liquid chromatography with ICP-MS and ESI-MS detection. Anal Bioanal Chem 407:7899–7906. https://doi.org/10.1007/s00216-015-8956-z
Chow CF, Lam MHW, Leung MKP (2002) Fluorescent sensing of homocysteine by molecular imprinting. Anal Chim Acta 466:17–30. https://doi.org/10.1016/S0003-2670(02)00520-2
Stachniuk J, Kubalczyk P, Furmaniak P, Gowacki R (2016) A versatile method for analysis of saliva, plasma and urine for total thiols using HPLC with UV detection. Talanta 155:70–77. https://doi.org/10.1016/j.talanta.2016.04.031
Borowczyk K, Chwatko G, Kubalczyk P, Jakubowski H, Kubalska J, Glowacki R (2016) Simultaneous determination of methionine and homocysteine by on column derivatization with o-phthalaldehyde. Talanta 161:917–924. https://doi.org/10.1016/j.talanta.2016.09.039
Accinni R, Bartesaghi S, De Leo G, Cursano CF, Achilli G, Loaldi A, Cellerino C, Parodi O (2000) Screening of homocysteine from newborn blood spots by high-performance liquid chromatography with coulometric array detection. J Chromatogr A 896:183–189. https://doi.org/10.1016/S0021-9673(00)00715-9
Magera MJ, Lacey JM, Bruno C, Piero R (1999) Method for the determination of total homocysteine in plasma and urine by stable isotope dilution and electrospray tandem mass spectrometry. Clin Chem 45:1517–1522. https://doi.org/10.1093/clinchem/45.9.1517
Kim MS, Cho S, Joo SH, Lee J, Kwak SK, Kim MI, Lee J (2019) N- and B- codoped graphene: a strong candidate to replace natural peroxidase in sensitive and selective bioassays. ACS Nano 13:4312–4321. https://doi.org/10.1021/acsnano.8b09519
Zhang T, Xing Y, Song Y, Gu Y, Yan X, Lu N, Liu H, Xu Z, Xu H, Zhang Z (2019) AuPt/MOF-graphene: a synergistic catalyst with surprisingly high peroxidase-like activity and its application for H2O2 detection. Anal Chem 91:10589–10595. https://doi.org/10.1021/acs.analchem.9b01715
Gao LZ, Zhuang J, Nie L, Zhang JB, Zhang Y, Gu N, Wang TH, Feng J, Yang DL, Perrett S, Yan X (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2:577–583. https://doi.org/10.1021/10.1038/nnano.2007.260
Liu CY, Miao YQ, Zhan XJ, Zhang SL, Zhao XJ (2020) Colorimetric determination of cysteine by a paper-based assay system using aspartic acid modified gold nanoparticles. Microchim Acta 187:362. https://doi.org/10.1007/s00604-020-04333-4
Xian Z, Zhang L, Yu Y, Lin B, Wang Y, Guo M, Cao Y (2021) Nanozyme based on CoFe2O4 modified with MoS2 for colorimetric determination of cysteine and glutathione. Microchim Acta 188:65. https://doi.org/10.1007/s00604-021-04702-7
Liang M, Yan X (2019) Nanozymes: from new concepts, mechanisms, and standards to applications. Accounts Chem Res 52:2190–2200. https://doi.org/10.1021/acs.accounts.9b00140
Chen W, Chen J, Feng YB, Hong L, Chen QY, Wu LF, Lin XH, Xia XH (2012) Peroxidase-like activity of water-soluble cupric oxide nanoparticles and its analytical application for detection of hydrogen peroxide and glucose. Analyst 137:1706–1712. https://doi.org/10.1021/10.1039/c2an35072f
Ruan X, Liu D, Niu X, Wang Y, Simpson CD, Cheng N, Du D, Lin Y (2019) 2D Graphene oxide/Fe-MOF nanozyme nest with superior peroxidase-like activity and its application for detection of woodsmoke exposure biomarker. Anal Chem 91:13847–13854. https://doi.org/10.1021/acs.analchem.9b03321
Fan K, Cao C, Pan Y, Lu D, Yang D, Feng J, Song L, Liang M, Yan X (2012) Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat Nanotechnol 7:833–U76. https://doi.org/10.1038/nnano.2012.90
Tarokh A, Pebdeni AB, Othman HO, Salehnia F, Hosseini M (2021) Sensitive colorimetric aptasensor based on g-C3N4@Cu2O composites for detection of Salmonella typhimurium in food and water. Microchim Acta 188:87. https://doi.org/10.1007/s00604-021-04745-w
Wang Q, Wei H, Zhang Z, Wang W, Dong S (2018) Nanozyme: an emerging alternative to natural enzyme for biosensing and immunoassay. TrAC-Trend Anal Chem 105:218–224. https://doi.org/10.1016/j.trac.2018.05.012
Guo J, Wu S, Wang Y, Zhao M (2020) A label-free fluorescence biosensor based on a bifunctional MIL-101(Fe) nanozyme for sensitive detection of choline and acetylcholine at nanomolar level. Sensor Actuat B-Chem 312:128021. https://doi.org/10.1016/j.snb.2020.128021
Zhang Z, Zhang X, Liu B, Liu J (2017) Molecular imprinting on inorganic nanozymes for hundred-fold enzyme specificity. J Am Chem Soc 139:5412–5419. https://doi.org/10.1021/jacs.7b00601
Barrow SJ, Kasera S, Rowland MJ, Barrio JD, Scherman OA (2015) Cucurbituril-based molecular recognition. Chem Rev 115:12320–12406. https://doi.org/10.1021/acs.chemrev.5b00341
Shcherbakova EG, Zhang B, Gozem S, Minami T, Zavalij PY, Pushina M, Isaacs L, Anzenbacher P (2017) Supramolecular sensors for opiates and their metabolites. J Am Chem Soc 139:14954–14960. https://doi.org/10.1021/jacs.7b06371
Berbeci LS, Wang W, Kaifer AE (2008) Drastically decreased reactivity of thiols and disulfides complexed by cucurbit[6]uril. Org Lett 10:3721–3724. https://doi.org/10.1021/ol8013667
Liu SD, Tian RZ, Xu JY, Wang L, Sun JX, Jiang XJ, Wang TT, Li XM, Luo Q, Liu JQ (2019) Cucurbit[8]uril-based supramolecular nanocapsules with a multienzyme-cascade antioxidative effect. Chem Commun 55:13820–13823. https://doi.org/10.1039/c9cc07085k
Jain Y, Kumari M, Singh RP, Kumar D, Gupta R (2020) Sonochemical decoration of graphene oxide with magnetic Fe3O4@CuO nanocomposite for efficient click synthesis of coumarin-sugar based bioconjugates and their cytotoxic activity. Cata Lett 150:1142–1154. https://doi.org/10.1007/s10562-019-02982-6
Zhang L, Hai X, Xia C, Chen X, Wang J (2017) Growth of CuO nanoneedles on graphene quantum dots as peroxidase mimics for sensitive colorimetric detection of hydrogen peroxide and glucose. Sensor Actuat B-Chem 248:374–384. https://doi.org/10.1016/j.snb.2017.04.011
Zeng Y, Cai W, Shao X (2015) Quantitative analysis of 17 amino acids in tobacco leaves using an amino acid analyzer and chemometric resolution. J Sep Sci 38:2053–2058. https://doi.org/10.1002/jssc.201500090
Wang R, Cui Y, Hu F, Liu W, Du Q, Zhang Y, Zha J, Huang T, Fizir M, He H (2019) Selective recognition and enrichment of carbamazepine in biological samples by magnetic imprinted polymer based on reversible addition-fragmentation chain transfer polymerization. J Chromatog A 1591:62–70. https://doi.org/10.1016/j.chroma.2019.01.057
Zheng S, Hu J, Zhong L, Song W, Wan L, Guo Y (2008) Introducing dual functional CNT networks into CuO nanomicrospheres toward superior electrode materials for lithium-ion batteries. Chem Mate 20:3617–3622. https://doi.org/10.1021/cm7033855
Qiu X, Zhou Y, Jin X, Qi A, Yang Y (2015) One-pot solvothermal synthesis of biocompatible magnetic nanoparticles mediated by cucurbit[n]urils. J Mater Chem C 3:3517–3521. https://doi.org/10.1039/C5TC00369E
Refsum H, Helland S, Ueland PM (1985) Radioenzymic determination of homocysteine in plasma and urine. Clin Chem 31:624–628. https://doi.org/10.1093/clinchem/31.4.624
Purgat K, Olejarz P, Koska I, Glowacki R, Kubalczyk P (2020) Determination of homocysteine thiolactone in human urine by capillary zone electrophoresis and single drop microextraction. Anal Biochem 596:113640. https://doi.org/10.1016/j.ab.2020.113640
Beitollahi H, Zaimbashi R, Mahani MT, Tajik S (2020) A label-free aptasensor for highly sensitive detection of homocysteine based on gold nanoparticles. Bioelectrochemistry 134:107497–107497. https://doi.org/10.1016/j.bioelechem.2020.107497
Xue H, Yu M, He K, Liu Y, Cao Y, Shui Y, Li J, Farooq M, Wang L (2020) A novel colorimetric and fluorometric probe for biothiols based on MnO2 NFs-rhodamine B system. Anal Chim Acta 1127:39–48. https://doi.org/10.1016/j.aca.2020.06.039
Funding
This work was financially supported by the Independent Innovation Fund Project of Agricultural Science and Technology of Jiangsu Province in 2017 (No. CX (17) 1003), the Natural Science Foundation of China Projects (No. 81950410634, No. QNJ20200010003) for a Foreign Youth Project fund, and Innovation and Entrepreneurship Training Program for Undergraduate (No. 202010316209).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 10479 kb)
Rights and permissions
About this article
Cite this article
Song, Z., Jiang, C., Wang, F. et al. Nanozyme based on graphene oxide modified with Fe3O4, CuO, and cucurbit[6]uril for colorimetric determination of homocysteine. Microchim Acta 188, 207 (2021). https://doi.org/10.1007/s00604-021-04868-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04868-0