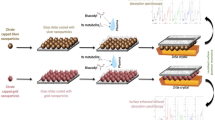
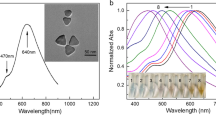
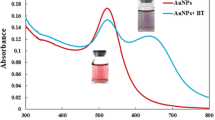
Abstract
Surface-enhanced infrared absorption spectroscopy offers an alternative to conventional IR spectroscopy and utilizes the signal enhancement exerted by the plasmon resonance of nanostructured metal thin films. Citrate-capped silver nanoparticles were prepared in a single-step method, and their morphology was identified using transmission electron microscopy, scanning electron microscopy, ultraviolet/visible spectrophotometry, and Zetasizer. The nanoparticles generated were deposited on the surface of cheap aluminum slides for different durations aiming for the selection of the best time producing a thin film, suitable to act as a lab-on-a-chip SEIRA substrate. These substrates were coupled to partial least squares regression tools for simultaneous resolving of the quinary mixture in commercial dosage forms of bisoprolol, perindopril, bisoprolol acid degradation product, bisoprolol alkali degradation product, and perindoprilat in concentration ranges of 15–75, 60–300, 15–55, 12–60, and 20–80 μg/mL with limits of detection values of 0.69, 3.43, 0.97, 1.25, and 1.09 μg/mL, respectively. Overall, we could demostrate that the localized surface plasmon resonance sensor coupled to chemometrics provides cheap, simple, selective, multiplex, rapid, and molecular specific procedures for impurity detection, which would be beneficial in many applications for quality control and quality accuracy of active pharmaceutical ingredients.
Graphical abstract







Similar content being viewed by others
References
Sala A, Anderson D, Brennan P, Butler H, Cameron J, Jenkinson M, Rinaldi C, Theakstone A, Baker M (2020) Biofluid diagnostics by FTIR spectroscopy: a platform technology for cancer detection. Cancer Lett 477:122–130
Eid SM, El-Rahman MKA, Elghobashy MR, Kelani KM (2017) Attenuated total reflectance Fourier transformation infrared spectroscopy fingerprinted online monitoring of the kinetics of circulating butyrylcholinesterase enzyme during metabolism of bambuterol. Anal Chim Acta
Granada E, Eguia P, Vilan J, Comesana J, Comesana R (2012) FTIR quantitative analysis technique for gases. Application in a biomass thermochemical process. Renew Energy 41:416–421
Lin M, Song Y, Lo P, Hsu C, Lin A, Huang E, Chiang H (2019) Quantitative analysis of calcium oxalate hydrate urinary stones using FTIR and 950/912 cm(−1) peak ratio. Vib Spectrosc 102:85–90
Reig F, Adelantado J, Moreno M (2002) FTIR quantitative analysis of calcium carbonate (calcite) and silica (quartz) mixtures using the constant ratio method. Application to geological samples. Talanta 58:811–821
Chophi R, Sharma S, Singh R (2020) Forensic analysis of red lipsticks using ATR-FTIR spectroscopy and chemometrics, forensic chemistry. 17
Kelani K, Rezk M, Monir H, ElSherbiny M, Eid S (2020) FTIR combined with chemometric tools (fingerprinting spectroscopy) in comparison to HPLC: which strategy offers more opportunities as a green analytical chemistry technique for pharmaceutical analysis. Anal Methods 12:5893–5907
Fanelli S, Zimmermann A, Totoli E, Salgado H (2018) FTIR spectrophotometry as a green tool for quantitative analysis of drugs: practical application to amoxicillin, journal of chemistry. 2018
Eid S, Kelani K, Badran O, Rezk M, Elghobashy M (2020) Surface enhanced infrared absorption spectroscopy (SEIRA) as a green analytical chemistry approach: coating of recycled aluminum TLC sheets with citrate capped silver nanoparticles for chemometric quantitative analysis of ternary mixtures as a green alternative to the traditional methods. Anal Chim Acta 1117:60–73
Wanzenböck HD, Mizaikoff B, Weissenbacher N, Kellner R (1997) Multiple internal reflection in surface enhanced infrared absorption spectroscopy (SEIRA) and its significance for various analyte groups. J Mol Struct 410-411:535–538
Mayerhöfer TG, Pipa AV, Popp J (2019) Beer's law-why integrated absorbance depends linearly on concentration. Chemphyschem 20:2748–2753
Algethami F, Eid S, Kelani K, Elghobashy M, Abd El-Rahman M (2020) Chemical fingerprinting and quantitative monitoring of the doping drugs bambuterol and terbutaline in human urine samples using ATR-FTIR coupled with a PLSR chemometric tool. RSC Adv 10:7146–7154
Hartstein A, Kirtley JR, Tsang JC (1980) Enhancement of the infrared absorption from molecular monolayers with thin metal overlayers. Phys Rev Lett 45:201–204
Aroca R, RodriguezLlorente S (1997) Surface-enhanced vibrational spectroscopy. J Mol Struct 408:17–22
Baia M, Toderas F, Baia L, Maniu D, Astilean S (2009) Multilayer structures of self-assembled gold nanoparticles as a unique SERS and SEIRA substrate. Chemphyschem 10:1106–1111
Bibikova O, Haas J, Lopez-Lorente A, Popov A, Kinnunen M, Meglinski I, Mizaikoff B (2017) Towards enhanced optical sensor performance: SEIRA and SERS with plasmonic nanostars. Analyst 142:951–958
Wedlich RC, Pires A, Fazzino L, Fransen JM (2013) Good laboratory practice. Part 3. Implementing good laboratory practice in the analytical lab. J Chem Educ 90:862–865
Hassan SA, Nashat NW, Elghobashy MR, Abbas SS, Moustafa AA (2020) Stability-indicating RP-HPLC and CE methods for simultaneous determination of bisoprolol and perindopril in pharmaceutical formulation: a comparative study. J Chromatogr Sci 58:747–758
Finkel R, Clark MA, Cubeddu LX (2009) Pharmacology. Lippincott Williams & Wilkins
A.C. Cartwright, The British pharmacopoeia, 1864 to 2014, 2016
Shaikh S, Thusleem OA, Muneera MS, Akmal J, Kondaguli AV, Ruckmani K (2008) A simple and rapid high-performance liquid chromatographic method for the determination of bisoprolol fumarate and hydrochlorothiazide in a tablet dosage form. J Pharm Biomed Anal 48:1055–1057
Zaazaa HE, Abbas SS, Essam HAM, El-Bardicy MG (2012) Validated chromatographic methods for determination of perindopril and amlodipine in pharmaceutical formulation in the presence of their degradation products. J Chromatogr Sci 51:533–543
Gkogkou D, Shaykhutdinov T, Kratz C, Oates TWH, Hildebrandt P, Weidinger IM, Ly KH, Esser N, Hinrichs K (2019) Gradient metal nanoislands as a unified surface enhanced Raman scattering and surface enhanced infrared absorption platform for analytics. Analyst 144:5271–5276
Brereton RG (2003) Chemometrics: data analysis for the laboratory and chemical plant. Wiley
Eid SM (2020) Indirect nano-sensing approach: a universal potentiometric silver ion selective sensor for inline quantitative profiling of the kinetics and thermodynamics of formation and decay of silver nanoparticles, Talanta, 218
Bastús NG, Merkoçi F, Piella J, Puntes V (2014) Synthesis of highly monodisperse citrate-stabilized silver nanoparticles of up to 200 nm: kinetic control and catalytic properties. Chem Mater 26:2836–2846
Agnihotri S, Mukherji S, Mukherji S (2013) Immobilized silver nanoparticles enhance contact killing and show highest efficacy: elucidation of the mechanism of bactericidal action of silver. Nanoscale 5
Pillai ZS, Kamat PV (2004) What factors control the size and shape of silver nanoparticles in the citrate ion reduction method? J Phys Chem B 108:945–951
Kurrey R, Deb MK, Shrivas K (2019) Surface enhanced infra-red spectroscopy with modified silver nanoparticles (AgNPs) for detection of quaternary ammonium cationic surfactants. New J Chem 43:8109–8121
Manojkumar K, Sivaramakrishna A, Vijayakrishna K (2016) A short review on stable metal nanoparticles using ionic liquids, supported ionic liquids, and poly(ionic liquids). J Nanopart Res 18
Kraynov A, Muller T, Handy S (2011) Concepts for the stabilization of metal nanoparticles in ionic liquids. Appl Ionic Liq Sci Technol:235–260
Otto A (2005) The 'chemical' (electronic) contribution to surface-enhanced Raman scattering. J Raman Spectrosc 36:497–509
Sperling RA, Parak WJ (2010) Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos Trans Math Phys Eng Sci 368:1333–1383
Lee LC, Liong C-Y, Jemain AA (2017) A contemporary review on data preprocessing (DP) practice strategy in ATR-FTIR spectrum. Chemom Intell Lab Syst 163:64–75
K.R. Beebe, R.J. Pell, M.B. Seasholtz, Chemometrics: a practical guide, Wiley New York, 1998
Donahue SM, Brown CW, Caputo B, Modell MD (1988) Near-infrared multicomponent analysis in the spectral and Fourier domains: energy content of high-pressure natural gas. Anal Chem 60:1873–1878
Haaland DM, Thomas EV (1988) Partial least-squares methods for spectral analyses. 1. Relation to other quantitative calibration methods and the extraction of qualitative information. Anal Chem 60:1193–1202
Kramer R (1998) Chemometric techniques for quantitative analysis. CRC Press
Haaland DM, Thomas EV (2002) Partial least-squares methods for spectral analyses. 1. Relation to other quantitative calibration methods and the extraction of qualitative information. Anal Chem 60:1193–1202
Abd El-Rahman M, Rezk M, Mahmoud A, Elghobashy M (2015) Design of a stable solid-contact ion-selective electrode based on polyaniline nanoparticles as ion-to-electron transducer for application in process analytical technology as a real-time analyzer. Sens Actuators B-Chem 208:14–21
Yan X, Li P, Zhou B, Tang X, Li X, Weng S, Yang L, Liu J (2017) Optimal hotspots of dynamic surfaced-enhanced Raman spectroscopy for drugs quantitative detection. Anal Chem 89:4875–4881
Zhao H, Hasi W, Bao L, Han S, Sha X, Sun J, Lou X, Lin D, Lv Z (2017) Rapid detection of sildenafil drugs in liquid nutraceuticals based on surface-enhanced Raman spectroscopy technology. Chin J Chem 35:1522–1528
Saleh T, Al-Shalalfeh M, Al-Saadi A (2018) Silver loaded graphene as a substrate for sensing 2-thiouracil using surface-enhanced Raman scattering. Sens Actuators B-Chem 254:1110–1117
Markina NE, Volkova EK, Zakharevich AM, Goryacheva IY, Markin AV (2018) SERS detection of ceftriaxone and sulfadimethoxine using copper nanoparticles temporally protected by porous calcium carbonate. Mikrochim Acta 185:481
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 1241 kb)
Rights and permissions
About this article
Cite this article
Eid, S.M., Hassan, S.A., Nashat, N.W. et al. Optimization of localized surface plasmon resonance hot spots in surface-enhanced infrared absorption spectroscopy aluminum substrate as an optical sensor coupled to chemometric tools for the purity assay of quinary mixtures. Microchim Acta 188, 195 (2021). https://doi.org/10.1007/s00604-021-04845-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04845-7