Abstract
Metal-organic framework (UiO-66-NH2)-incorporated organic polymer monolith was prepared by thermal polymerization. By virtue of the superior physical and chemical properties, the UiO-66-NH2-modified organic monolith was then functionalized by chiral selector cellulase via the condensation reaction between the primary amino groups and aldehyde groups. The synthesized materials were characterized by Fourier transform infrared spectroscopy, high-resolution transmission electron microscopy, scanning electron microscopy, X-ray photoelectron spectrometry, thermogravimetric analysis, and nitrogen sorption isotherm. The cellulase@poly(glycidyl methacrylate-UiO-66-NH2-ethylene glycol dimethacrylate) (cellulase@poly(GMA-UiO-66-NH2-EDMA)) monolith was applied to enantiomerically separate the basic racemic forms of metoprolol, atenolol, esmolol, bisoprolol, and propranolol. In contrast to the cellulase@poly(GMA-co-EDMA) monolith without UiO-66-NH2, the cellulase@poly(GMA-UiO-66-NH2-EDMA) monolith reveals significantly improved enantiodiscrimination performance for metoprolol (Rs: 0 → 1.67), atenolol (Rs: 0 → 1.50), esmolol (Rs: 0 → 1.52), bisoprolol (Rs: 0 → 0.36), and propranolol (Rs: 0 → 0.44). The immobilization pH of cellulase, buffer pH, UiO-66-NH2 concentration, and the proportion of organic modifier were evaluated in detail with enantiomerically separating chiral molecules. The intra-day, inter-day, column-to-column, and inter-batch precision have been discussed, the result was preferable, and the relative standard deviation (RSD) of separation parameters was <4.3%.
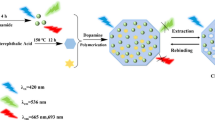
Graphical abstract

Schematic representation of the preparation of a UiO-66-NH2-modified organic polymer monolith for enantioseparating five racemic β-blockers. UiO-66-NH2 was synthesized and converted into a monolith as the stationary phase. Then, the modified monolith containing cellulase as the chiral selector was applied in a capillary electrochromatography system for enantioseparating chiral drugs.





Similar content being viewed by others
References
Michaeli K, Kantor-Uriel N, Naaman R, Waldeck DH (2016) The electron’s spin and molecular chirality-how are they related and how do they affect life processes. Chem Soc Rev 45:6478–6487. https://doi.org/10.1039/C6CS00369A
Zheng Z, Lin J, Qu F (2003) Chiral separation of underivatized and dansyl amino acids by ligand-exchange micellar electrokinetic capillary chromatography using a copper (II)-L-valine complex as selector. J Chromatogr A 1007:189–196. https://doi.org/10.1016/S0021-9673(03)00960-9
Stalcup A (2010) Chiral separations. Annu Rev Anal Chem 3:341–363. https://doi.org/10.1146/annurev.anchem.111808.073635
Scriba G (2012) Chiral recognition mechanisms in analytical separation sciences. Chromatographia 75:815–838. https://doi.org/10.1016/j.chroma.2016.05.061
Banerjee-Ghosh K, Dor O, Tassinari F, Capua E, Yochelis S, Capua A, Yang S, Parkin S, Sarkar S, Kronik L, Baczewski L, Naaman R, Paltie Y (2018) Separation of enantiomers by their enantiospecific interaction with achiral magnetic substrates. Science 360:1331–1334. https://doi.org/10.1126/science.aar4265
Shi H, Herron A, Shao Y, Shao Q, Yu J (2018) Enantioselective remote meta-C-H arylation and alkylation via a chiral transient mediator. Nature 558:581–585. https://doi.org/10.1038/s41586-018-0220-1
Tan H, Liu T, Zhang X, Shan Q, Chen J, Li Z, Ihara H, Qiu H (2020) Preparation of vortex porous graphene chiral membrane for enantioselective separation. Anal Chem 92:13630–13633. https://doi.org/10.1021/acs.analchem.0c02446
Svec F, Lv Y (2015) Advances and recent trends in the field of monolithic columns for chromatography. Anal Chem 87:250–273. https://doi.org/10.1021/ac504059c
Hong T, Yang X, Xu Y, Ji Y (2016) Recent advances in the preparation and application of monolithic capillary columns in separation science. Anal Chim Acta 931:1–24. https://doi.org/10.1016/j.aca.2016.05.013
Teixeira J, Tiritan M, Pinto M, Fernandes C (2019) Chiral stationary phases for liquid chromatography: recent developments. Molecules 24:865. https://doi.org/10.3390/molecules24050865
Xie S, Yuan L (2019) Recent development trends for chiral stationary phases based on chitosan derivatives, cyclofructan derivatives and chiral porous materials in high performance liquid chromatography. J Sep Sci 42:6–20. https://doi.org/10.1002/jssc.201800656
Jiang H, Yang K, Zhao X, Zhang W, Liu Y, Jiang J, Cui Y (2021) Highly stable Zr (IV)-based metal-organic frameworks for chiral separation in reversed-phase liquid chromatography. J Am Chem Soc 143:390–398. https://doi.org/10.1021/jacs.0c11276
Li J, Bhatt P, Li J, Eddaoudi M, Liu Y (2020) Recent progress on microfine design of metal–organic frameworks: structure regulation and gas sorption and separation. Adv Mater 32:2002563. https://doi.org/10.1002/adma.202002563
Wang L, Yang C, Yan X (2017) In situ growth of covalent organic framework shells on silica microspheres for application in liquid chromatography. ChemPlusChem 82:933–938. https://doi.org/10.1002/cplu.201700223
Zhang K, Cai S, Yan Y, He Z, Lin H, Huang X, Zheng S, Fan J, Zhang W (2017) Construction of a hydrazone-linked chiral covalent organic framework-silica composite as the stationary phase for high performance liquid chromatography. J Chromatogr A 1519:100–109. https://doi.org/10.1016/j.chroma.2017.09.007
Zhang J, Zhu P, Xie S, Zi M, Yuan L (2018) Homochiral porous organic cage used as stationary phase for open tubular capillary electrochromatography. Anal Chim Acta 999:169–175. https://doi.org/10.1016/j.aca.2017.11.021
Dong J, Liu Y, Cui Y (2014) Chiral porous organic frameworks for asymmetric heterogeneous catalysis and gas chromatographic separation. Chem Commun. (Cambridge, U. K.) 50:14949–14952. https://doi.org/10.1039/c4cc07648f
Yaghi O, Li H (1995) Hydrothermal synthesis of a metal-organic framework containing large rectangular channels. J Am Chem Soc 117:10401–10402. https://doi.org/10.1021/ja00146a033
Li B, Wen H, Zhou W, Chen B (2014) Porous metal-organic frameworks for gas storage and separation: what, how, and why? J Phys Chem Lett 5:3468–3479. https://doi.org/10.1021/jz501586e
Yang X, Xu Q (2017) Bimetallic metal-organic frameworks for gas storage and separation. Cryst Growth Des 17:1450–1455. https://doi.org/10.1021/acs.cgd.7b00166
Li J, Sculley J, Zhou H (2012) Metal-organic frameworks for separations. Chem Rev 112:869–932. https://doi.org/10.1021/cr200190s
Farrusseng D, Aguado S, Pinel C (2009) Metal-organic frameworks: opportunities for catalysis. Angew Chem Int Ed 48:7502–7513. https://doi.org/10.1002/anie.200806063
Zhao M, Ou S, Wu C (2014) Porous metal-organic frameworks for heterogeneous biomimetic catalysis. Acc Chem Res 47:1199–1207. https://doi.org/10.1021/ar400265x
Rojas S, Wheatley P, Quartapelle-Procopio E, Gil B, Marszalek B, Morris R, Barea E (2013) Metal-organic frameworks as potential multi-carriers of drugs. Crystengcomm 15:9364–9367. https://doi.org/10.1039/C3CE41289J
Della Rocca J, Liu D, Lin W (2011) Nanoscale metal-organic frameworks for biomedical imaging and drug delivery. Acc Chem Res 44:957–968. https://doi.org/10.1021/ar200028a
Cavka J, Jakobsen S, Olsbye U, Guillou N, Lamberti C, Bordiga S, Lillerud K (2008) A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J Am Chem Soc 130:3850–13851. https://doi.org/10.1021/ja8057953
Kandiah M, Nilsen M, Usseglio S, Jakobsen S, Olsbye U, Tilset M, Larabi C, Quadrelli E, Bonino F, Lillerud K (2010) Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem Mater 22:6632–6640. https://doi.org/10.1021/cm102601v
Kim M, Cohen S (2012) Discovery, development, and functionalization of Zr(IV)-based metal-organic frameworks. Crystengcomm 14:4096–4104. https://doi.org/10.1039/c2ce06491j
Zhao W, Zhang C, Yan Z, Bai L, Wang X, Huang H, Zhou Y, Xie Y, Li F, Li J (2014) Separations of substituted benzenes and polycyclic aromatic hydrocarbons using normal- and reverse-phase high performance liquid chromatography with UiO-66 as the stationary phase. J Chromatogr A 1370:121–128. https://doi.org/10.1016/j.chroma.2014.10.036
Fu Y, Yang C, Yan X (2013) Incorporation of metal-organic framework UiO-66 into porous polymer monoliths to enhance the liquid chromatographic separation of small molecules. Chem Commun 49:7162–7164. https://doi.org/10.1039/c3cc43017k
Chang N, Yan X (2012) Exploring reverse shape selectivity and molecular sieving effect of metal-organic framework UIO-66 coated capillary column for gas chromatographic separation. J Chromatogr A 1257:116–124. https://doi.org/10.1016/j.chroma.2012.07.097
Zhang Y, Lynd L (2004) Toward an aggregated understanding of enzymatic hydrolysis of cellulose: noncomplexed cellulase systems. Biotechnol Bioeng 88:797–824. https://doi.org/10.1002/bit.20282
Matsunaga H, Haginaka J (2016) Separation of enantiomers on chiral stationary phase based on cellulase: effect of preparation method and silica particle diameters on chiral recognition ability. J Chromatogr A 1467:155–162. https://doi.org/10.1016/j.chroma.2016.05.069
Liu Z, Du Y, Feng Z (2017) Enantioseparation of drugs by capillary electrochromatography using a stationary phase covalently modified with graphene oxide. Microchim Acta 184:583–593. https://doi.org/10.1007/s00604-016-2014-1
Ma M, Du Y, Yang J, Feng Z, Ding W, Chen C (2020) Gold nanoparticles-functionalized monolithic column for enantioseparation of eight basic chiral drugs by capillary electrochromatography. Microchim Acta 187:178. https://doi.org/10.1007/s00604-020-4144-8
Hou J, Luan Y, Tang J, Wensley A, Yang M, Lu Y (2015) Synthesis of UiO-66-NH2 derived heterogeneous copper (II) catalyst and study of its application in the selective aerobic oxidation of alcohols. J Mol Catal A Chem 407:53–59. https://doi.org/10.1016/j.molcata.2015.06.018
Tang J, Dong W, Wang G, Yao Y, Cai L, Liu Y, Zhao X, Xu J, Tan L (2014) Efficient molybdenum (VI) modified Zr-MOF catalysts for epoxidation of olefins. RSC Adv 4:42977–42982. https://doi.org/10.1039/c4ra07133f
Du Y, Li X, Lv X, Jia Q (2017) Highly sensitive and selective sensing of free bilirubin using metal-organic frameworks-based energy transfer process. ACS Appl Mater Interfaces 9:30925–30932. https://doi.org/10.1021/acsami.7b09091
Pintado-Sierra M, Rasero-Almansa A, Corma A, Iglesias M, Sánchez F (2013) Bifunctional iridium-(2-aminoterephthalate)-Zr-MOF chemoselective catalyst for the synthesis of secondary amines by one-pot three-step cascade reaction. J Catal 299:137–145. https://doi.org/10.1016/j.jcat.2012.12.00
Azhar M, Abid H, Periasamy V, Sun H, Tade M, Wang S (2017) Adsorptive removal of antibiotic sulfonamide by UiO-66 and ZIF-67 for wastewater treatment. J Colloid Interface Sci 500:88–95. https://doi.org/10.1016/j.jcis.2017.04.001
Hu Z, Peng Y, Kang Z, Qian Y, Zhao D (2015) A modulated hydrothermal (MHT) approach for the facile synthesis of UiO-66-type MOFs. Inorg Chem 54:4862–4868. https://doi.org/10.1021/acs.inorgchem.5b00435
Hu P, Liang X, Yaseen M, Sun X, Tong Z, Zhao Z, Zhao Z (2018) Preparation of highly-hydrophobic novel N-coordinated UiO-66(Zr) with dopamine via fast mechano-chemical method for (CHO−/cl−)-VOCs competitive adsorption in humid environment. Chem Eng J 332:608–618. https://doi.org/10.1016/j.cej.2017.09.115
Yang Z, Tong X, Feng J, He S, Fu M, Niu X, Zhang T, Liang H, Ding A, Feng X (2019) Flower-like BiOBr/UiO-66-NH2 nanosphere with improved photocatalytic property for norfloxacin removal. Chemosphere 220:98–106. https://doi.org/10.1016/j.chemosphere.2018.12.086
He T, Xu X, Ni B, Wang H, Long Y, Hu W, Wang X (2017) Fast and scalable synthesis of uniform zirconium, hafnium-based metal-organic framework nanocrystals. Nanoscale 9:19209–19215. https://doi.org/10.1039/c7nr06274e
Wang T, Wang Y, Zhang Y, Cheng Y, Ye J, Chu Q, Cheng G (1625) Rapid preparation and evaluation of chiral open-tubular columns supported with bovine serum album and zeolite imidazolate framework-8 for mini-capillary electrochromatography. J Chromatogr A 2020:461284. https://doi.org/10.1016/j.chroma.2020.461284
Hong T, Chen X, Xu Y, Cui X, Bai R, Jin C, Li R, Ji Y (2016) Preparation of graphene oxide-modified affinity capillary monoliths based on three types of amino donor for chiral separation and proteolysis. J Chromatogr A 1456:249–256. https://doi.org/10.1016/j.chroma.2016.06.025
Funding
This work was supported by the Project of National Natural Science Foundation of China (No.:82073809).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 1.13 mb)
Rights and permissions
About this article
Cite this article
Ma, M., Zhang, J., Li, P. et al. Immobilization of cellulase on monolith supported with Zr(IV)-based metal-organic framework as chiral stationary phase for enantioseparation of five basic drugs in capillary electrochromatography. Microchim Acta 188, 186 (2021). https://doi.org/10.1007/s00604-021-04840-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04840-y