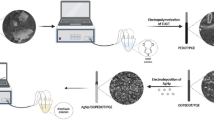
Abstract
Lithium cobalt phosphate (LCP) was prepared and modified on the surface of glassy carbon electrode (GCE) to fabricate the electrochemical sensor (LCP/GCE) for the simultaneous determination of ascorbic acid (AA), dopamine (DA), and serum uric acid (UA). The homogenous incorporation of carbon improved the conductivity of LCP. Benefiting from the small particle size distribution, LCP/GCE has a large active surface and responds to AA, DA, and UA sensitively and rapidly. For the simultaneous detection with differential pulse voltammetry the anodic peaks of AA, DA, and UA were well-separated and appeared at ~0 V, ~0.19 V, and ~ 0.33 V (vs. Ag/AgCl), respectively. The linear responses toward AA, DA, and UA were in the range 10 μM–8.0 mM, 10 nM–10 μM, and 0.020 μM–25 μM; the detection limits were estimated to be 8.10 μM, 7.50 nM, and 22.7 nM (S/N = 3), respectively. The excellent selectivity and reproducibility of LCP/GCE enable serum UA to be detected without the interference of AA and DA. The recoveries of DA and AA in the serum sample were in the range 95 to 111%. The results indicate that LCP has the potential to be developed as the sensing devices to be applied to in vitro diagnosis.
Graphical abstract

The lithium-ion battery cathodic material, LCP with the excellent adsorption and catalytic behavior, was utilized to fabricate the electrochemical sensor for the sensitive and simultaneous detection of AA, DA, and UA, which achieved the low detection limits and the wide concentration ranges. LCP/GCE can be used for the quantitative detection of serum UA without the interference of DA and AA. In addition, the recoveries of DA and AA in human serum were satisfactory, which illustrate the reliability of LCP/GCE to be applied to in vitro diagnosis.









Similar content being viewed by others
References
Sajid M, Nazal MK, Mansha M, Alsharaa A, Jillani SMS, Basheer C (2016) Chemically modified electrodes for electrochemical detection of dopamine in the presence of uric acid and ascorbic acid: a review. TrAC Trends Anal Chem 76:15–29
Zhao J, Wang S, Lu S, Bao X, Sun J, Yang X (2018) An enzyme cascade-triggered fluorogenic and chromogenic reaction applied in enzyme activity assay and immunoassay. Anal Chem 90(12):7754–7760
Yildirim A, Bayindir M (2014) Turn-on fluorescent dopamine sensing based on in situ formation of visible light emitting polydopamine nanoparticles. Anal Chem 86(11):5508–5512
Lan Y, Yuan F, Fereja TH, Wang C, Lou B, Li J, Xu G (2019) Chemiluminescence of lucigenin/riboflavin and its application for selective and sensitive dopamine detection. Anal Chem 91(3):2135–2139
Zhang L, Cheng Y, Lei J, Liu Y, Hao Q, Ju H (2013) Stepwise chemical reaction strategy for highly sensitive electrochemiluminescent detection of dopamine. Anal Chem 85(16):8001–8007
Li D, Liu M, Zhan Y, Su Q, Zhang Y, Zhang D (2020) Electrodeposited poly(3,4-ethylenedioxythiophene) doped with graphene oxide for the simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid. Microchim Acta 187(1):94
Hu K, Wang D, Zhou M, Bae JH, Yu Y, Xin H, Mirkin MV (2019) Ultrasensitive detection of dopamine with carbon nanopipets. Anal Chem 91(20):12935–12941
Du J, Yue R, Ren F, Yao Z, Jiang F, Yang P, Du Y (2014) Novel graphene flowers modified carbon fibers for simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens Bioelectron 53:220–224
Asif M, Aziz A, Wang H, Wang Z, Wang W, Ajmal M, Xiao F, Chen X, Liu H (2019) Superlattice stacking by hybridizing layered double hydroxide nanosheets with layers of reduced graphene oxide for electrochemical simultaneous determination of dopamine, uric acid and ascorbic acid. Microchim Acta 186(2):61
Yao Y, Zhong J, Lu Z, Liu X, Wang Y, Liu T, Zou P, Dai X, Wang X, Ding F, Zhou C, Zhao Q, Rao H (2019) Nitrogen-doped carbon frameworks decorated with palladium nanoparticles for simultaneous electrochemical voltammetric determination of uric acid and dopamine in the presence of ascorbic acid. Microchim Acta 186(12):795
Li Y, Lin H, Peng H, Qi R, Luo C (2016) A glassy carbon electrode modified with MoS2 nanosheets and poly(3,4-ethylenedioxythiophene) for simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid. Microchim Acta 183(9):2517–2523
Min Z, Nuria GA, Andrew LH (2018) Understanding and development of olivine LiCoPO4 cathode materials for lithium-ion batteries. J Mater Chem A 6:14483–14517
Kang YM, Kim YI, Oh MW, Yin RZ, Lee Y, Han DW, Kwon HS, Kim JH, Ramanath G (2011) Structurally stabilized olivine lithium phosphate cathodes with enhanced electrochemical properties through fe doping. Energy Environ Sci 4(12):4978–4983
Xu Y, Gao T, Liang Y, Xiao D (2020) Intercalation lithium cobalt oxide for the facile fabrication of a sensitive dopamine sensor. ChemElectroChem 7(5):1193–1200
Li Y, Zhao C (2017) Enhancing water oxidation catalysis on a synergistic phosphorylated NiFe hydroxide by adjusting catalyst wettability. ACS Catal 7(4):2535–2541
Yuan L, Wang Z, Zhang WX, Hu X, Chen J, Huang Y, Goodenough JB (2011) Development and challenges of LiFePO4 cathode material for lithium-ion batteries. Energy Environ Sci 4(2):269–284
Örnek A (2017) An impressive approach to solving the ongoing stability problems of LiCoPO4 cathode: nickel oxide surface modification with excellent core-shell principle. J Power Sources 356:1–11
Zhang Y, Chen L, Ou J, Wang J, Zheng B, Yuan H, Guo Y, Xiao D (2013) Improving the performance of a LiFePO4 cathode based on electrochemically cleaved graphite oxides with high hydrophilicity and good conductivity. J Mater Chem A 1(27):7933–7941
Doan TNL, Taniguchi I (2011) Preparation of LiCoPO4/C nanocomposite cathode of lithium batteries with high rate performance. J Power Sources 196(13):5679–5684
Sharabi R, Markevich E, Fridman K, Gershinsky G, Salitra G, Aurbach D, Semrau G, Schmidt MA, Schall N, Bruenig C (2013) Electrolyte solution for the improved cycling performance of LiCoPO4/C composite cathodes. Electrochem Commun 28:20–23
Chen X, Li P, Jin Z, Meng Y, Yuan H, Xiao D (2017) Tri-metallic phytate in situ electrodeposited on 3d Ni foam as a highly efficient electrocatalyst for enhanced overall water splitting. J Mater Chem A 5(35):18786–18792
Maeyoshi Y, Miyamoto S, Munakata H, Kanamura K (2018) Effect of conductive carbon additives on electrochemical performance of LiCoPO4. J Power Sources 376:18–25
Menezes PW, Indra A, Zaharieva I, Walter C, Loos S, Hoffmann S, Schlögl R, Dau H, Driess M (2019) Helical cobalt borophosphates to master durable overall water-splitting. Energy Environ Sci 12(3):988–999
Dai L, Qin Q, Zhao X, Xu C, Hu C, Mo S, Wang YO, Lin S, Tang Z, Zheng N (2016) Electrochemical partial reforming of ethanol into ethyl acetate using ultrathin Co3O4 nanosheets as a highly selective anode catalyst. ACS Cent Sci 2(8):538–544
Muguruma H, Inoue Y, Inoue H, Ohsawa T (2016) Electrochemical study of dopamine at electrode fabricated by cellulose-assisted aqueous dispersion of long-length carbon nanotube. J Phys Chem C 120(22):12284–12292
Lee H, Dellatore SM, Miller WM, Messersmith PB (2007) Mussel-inspired surface chemistry for multifunctional coatings. Science 318(5849):426–430
Patel AN, Tan SY, Miller TS, Macpherson JV, Unwin PR (2013) Comparison and reappraisal of carbon electrodes for the voltammetric detection of dopamine. Anal Chem 85(24):11755–11764
Zhao Y, Gao Y, Zhan D, Liu H, Zhao Q, Kou Y, Shao Y, Li M, Zhuang Q, Zhu Z (2005) Selective detection of dopamine in the presence of ascorbic acid and uric acid by a carbon nanotubes-ionic liquid gel modified electrode. Talanta 66(1):51–57
Chen CT, Martin-Martinez FJ, Jung GS, Buehler MJ (2017) Polydopamine and eumelanin molecular structures investigated with ab initio calculations. Chem Sci 8(2):1631–1641
Wang L, Yang R, Qu L, Harrington PB (2020) Electrostatic repulsion strategy for high-sensitive and selective determination of dopamine in the presence of uric acid and ascorbic acid. Talanta 210:120626
Anson FC (1964) Application of potentiostatic current integration to the study of the adsorption of cobalt(III)-(ethylenedinitrilo(tetraacetate) on mercury electrodes. Anal Chem 36(4):932–934
Wang G, Meng J, Liu H, Jiao S, Zhang W, Chen D, Fang B (2008) Determination of uric acid in the presence of ascorbic acid with hexacyanoferrate lanthanum film modified electrode. Electrochim Acta 53(6):2837–2843
Dinesh B, Saraswathi R, Senthil Kumar A (2017) Water based homogenous carbon ink modified electrode as an efficient sensor system for simultaneous detection of ascorbic acid, dopamine and uric acid. Electrochim Acta 233:92–104
Jiang J, Du X (2014) Sensitive electrochemical sensors for simultaneous determination of ascorbic acid, dopamine, and uric acid based on Au@Pd-reduced graphene oxide nanocomposites. Nanoscale 6(19):11303–11309
Arroquia A, Acosta I, Armada MPG (2020) Self-assembled gold decorated polydopamine nanospheres as electrochemical sensor for simultaneous determination of ascorbic acid, dopamine, uric acid and tryptophan. Mater Sci Eng C 109:110602
Acknowledgements
Thanks to West China Hospital for the supply of serum samples.
Funding
This work was supported by the Major Instrument Project of the National Natural Science Foundation of China (Grant No. 81927809).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors. In this experiment, human serum samples were received from the West China Hospital, Sichuan University.
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no competing of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 9855 kb)
Rights and permissions
About this article
Cite this article
Xu, Y., Meng, Z., Meng, Y. et al. Lithium cobalt phosphate electrode for the simultaneous determination of ascorbic acid, dopamine, and serum uric acid by differential pulse voltammetry. Microchim Acta 188, 190 (2021). https://doi.org/10.1007/s00604-021-04839-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04839-5