Abstract
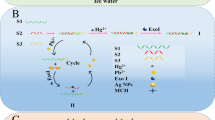
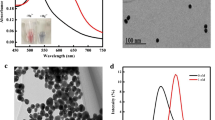
A reagent-less DNA sensor has been developed exploiting a combination of gold nanoparticles, modified primers, and isothermal amplification. It is applied to the determination ofKarlodinium armiger, a toxic microalgae, as a model analyte to demonstrate this generic platform. Colloidal gold nanoparticles with an average diameter of 14 ± 0.87 nm were modified with a mixed self-assembled monolayer of thiolated 33-mer DNA probes and (6-mercaptohexyl) ferrocene. Modified primers, exploiting a C3 spacer between the primer-binding site and an engineered single-stranded tail, were used in an isothermal recombinase polymerase amplification reaction to produce an amplicon by two single-stranded tails. These tails were designed to be complementary to a gold electrode tethered capture oligo probe, and an oligo probe immobilized on the gold nanoparticles, respectively. The time required for hybridization of the target tailed DNA with the surface immobilized probe and reporter probe immobilized on AuNPs was optimized and reduced to 10 min, in both cases. Amplification time was further optimized to be 40 min to ensure the maximum signal. Under optimal conditions, the limit of detection was found to be 1.6 fM of target dsDNA. Finally, the developed biosensor was successfully applied to the detection of genomic DNA extracted from a seawater sample that had been spiked with K. armiger cells. The demonstrated generic electrochemical genosensor can be exploited for the detection of any DNA sequence and ongoing work is moving towards an integrated system for use at the point-of-need.







Similar content being viewed by others
Data availability
Not
References
Rafique B, Iqbal M, Mehmood T, Shaheen MA (2019) Electrochemical DNA biosensors: a review. Sens Rev 39:34–50. https://doi.org/10.1108/SR-08-2017-0156
Lobato IM, O’Sullivan CK (2018) Recombinase polymerase amplification: basics, applications and recent advances. TrAC Trends Anal Chem 98:19–35. https://doi.org/10.1016/j.trac.2017.10.015
Tan L, Ge J, Jiao M, Jie G, Niu S (2018) Amplified electrochemiluminescence detection of DNA based on novel quantum dots signal probe by multiple cycling amplification strategy. Talanta 183:108–113. https://doi.org/10.1016/j.talanta.2018.02.063
Mousavisani SZ, Raoof JB, Turner APF, Ojani R, Mak WC (2018) Label-free DNA sensor based on diazonium immobilisation for detection of DNA damage in breast cancer 1 gene. Sensors Actuators B Chem 264:59–66. https://doi.org/10.1016/j.snb.2018.02.152
Joda H, Beni V, Willems A, Frank R, Höth J, Lind K, Strömbom L, Katakis I, O´Sullivan CK (2015) Modified primers for rapid and direct electrochemical analysis of coeliac disease associated HLA alleles. Biosens Bioelectron 73:64–70. https://doi.org/10.1016/j.bios.2015.05.048
Al-Madhagi S, Joda H, Jauset-Rubio M et al (2018) Isothermal amplification using modified primers for rapid electrochemical analysis of coeliac disease associated DQB1*02 HLA allele. Anal Biochem 556:16–22. https://doi.org/10.1016/j.ab.2018.06.013
Mayboroda O, Benito AG, Del Rio JS et al (2016) Isothermal solid-phase amplification system for detection of Yersinia pestis. Anal Bioanal Chem 408:671–676. https://doi.org/10.1007/s00216-015-9177-1
Del Río JS, Svobodova M, Bustos P et al (2016) Electrochemical detection of Piscirickettsia salmonis genomic DNA from salmon samples using solid-phase recombinase polymerase amplification. Anal Bioanal Chem 408:8611–8620. https://doi.org/10.1007/s00216-016-9639-0
Del Río JS, Yehia Adly N, Acero-Sánchez JL et al (2014) Electrochemical detection of francisella tularensis genomic DNA using solid-phase recombinase polymerase amplification. Biosens Bioelectron 54:674–678. https://doi.org/10.1016/j.bios.2013.11.035
Del Río JS, Lobato IM, Mayboroda O et al (2017) Enhanced solid-phase recombinase polymerase amplification and electrochemical detection. Anal Bioanal Chem 409(12):3261–3269. https://doi.org/10.1007/s00216-017-0269-y
Ahmed N, AL-Madhagi S, Ortiz M et al (2020) Direct electrochemical detection of enzyme labelled, isothermally amplified DNA. Anal Biochem 598:113705. https://doi.org/10.1016/j.ab.2020.113705
Reta N, Saint CP, Michelmore A, Prieto-Simon B, Voelcker NH (2018) Nanostructured electrochemical biosensors for label- free detection of water- and food-borne pathogens. ACS Appl Mater Interfaces 10(7):6055–6072. https://doi.org/10.1021/acsami.7b13943
Wang Y, Sauriat-Dorizon H, Korri-Youssoufi H (2017) Direct electrochemical DNA biosensor based on reduced graphene oxide and metalloporphyrin nanocomposite. Sensors Actuators B Chem 251:40–48. https://doi.org/10.1016/j.snb.2017.04.128
Teengam P, Siangproh W, Tuantranont A, Vilaivan T, Chailapakul O, Henry CS (2018) Electrochemical impedance-based DNA sensor using pyrrolidinyl peptide nucleic acids for uberculosis detection. AC SC. Anal Chim Acta 1044:102–109. https://doi.org/10.1016/j.aca.2018.07.045
Bizid S, Mlika R, Haj Said A, Chemli M, Korri Youssoufi H (2016) Investigations of poly(p-phenylene) modified with ferrocene and their application in electrochemical DNA sensing. Sensors Actuators B Chem 226:370–380. https://doi.org/10.1016/j.snb.2015.11.137
Liu H, Bei X, Xia Q, Fu Y, Zhang S, Liu M, Fan K, Zhang M, Yang Y (2016) Enzyme-free electrochemical detection of microRNA-21 using immobilized hairpin probes and a target-triggered hybridization chain reaction amplification strategy. Microchim Acta 183:297–304. https://doi.org/10.1007/s00604-015-1636-z
Pearce DM, Shenton DP, Holden J, Gaydos CA (2011) Evaluation of a novel electrochemical detection method for chlamydia trachomatis: application for point-of-care diagnostics. IEEE Trans Biomed Eng 58:755–758. https://doi.org/10.1109/TBME.2010.2095851
Jin X, Zhou L, Zhu B, Jiang X, Zhu N (2018) Silver-dendrimer nanocomposites as oligonucleotide labels for electrochemical stripping detection of DNA hybridization. Biosens Bioelectron 107:237–243. https://doi.org/10.1016/j.bios.2018.02.033
Wang Y, Meng W, Chen X, Zhang Y (2020) DNA-templated copper nanoparticles as signalling probe for electrochemical determination of microRNA-222. Microchim Acta 187:4. https://doi.org/10.1007/s00604-019-4011-7
De La Escosura-Muñiz A, Baptista-Pires L, Serrano L et al (2016) Magnetic bead/gold nanoparticle double-Labeled primers for electrochemical detection of isothermal amplified Leishmania DNA. Small 12:205–213. https://doi.org/10.1002/smll.201502350
Toldrà A, Andree KB, Fernández-tejedor M et al (2018) Dual quantitative PCR assay for identification and enumeration of Karlodinium veneficum and Karlodinium armiger combined with a simple and rapid DNA extraction method. J Appl Phycol 30:2435–2445. https://doi.org/10.1007/s10811-018-1446-x
Joda H, Valerio H, Noora A, et al(2014) Medium-high resolution electrochemical genotyping of HLA-DQ2 / DQ8 for detection of predisposition to coeliac disease. Anal Bioanal Chem. 406:2757–27692757–2769. https://doi.org/10.1007/s00216-014-7650-x
Acero Sánchez JL, Joda H, Henry OYF, Solnestam BW, Kvastad L, Akan PS, Lundeberg J, Laddach N, Ramakrishnan D, Riley I, Schwind C, Latta D, O’Sullivan CK (2017) Electrochemical genetic profiling of single cancer cells. Anal Chem 89:3378–3385. https://doi.org/10.1021/acs.analchem.6b03973
Liu X, Atwater M, Wang J, Huo Q (2007) Extinction coefficient of gold nanoparticles with different sizes and different capping ligands. Colloids Surf B: Biointerfaces 58:3–7. https://doi.org/10.1016/j.colsurfb.2006.08.005
Liu L, Pan H, Du M et al (2010) Glassy carbon electrode modified with nafion-au colloids for clenbuterol electroanalysis. Electrochim Acta 55:7240–7245. https://doi.org/10.1016/j.electacta.2010.06.078
Shi A, Wang J, Han X, Fang X, Zhang Y (2014) A sensitive electrochemical DNA biosensor based on gold nanomaterial and graphene amplified signal. Sensors Actuators B Chem 200:206–212. https://doi.org/10.1016/j.snb.2014.04.024
Lau HY, Wu H, Wee EJH, Trau M, Wang Y, Botella JR (2017) Specific and sensitive isothermal electrochemical biosensor for plant pathogen DNA detection with colloidal gold nanoparticles as probes. Sci Rep 7:1–7. https://doi.org/10.1038/srep38896
Han S, Liu W, Zheng M, Wang R (2020) Label-free and ultrasensitive electrochemical dna biosensor based on urchinlike carbon nanotube-gold nanoparticle nanoclusters. Anal Chem 92:4780–4787. https://doi.org/10.1021/acs.analchem.9b03520
Sun H, Kong J, Wang Q, Liu Q, Zhang X (2019) Dual signal amplification by eATRP and DNA-templated silver nanoparticles for ultrasensitive electrochemical detection of nucleic acids. ACS Appl Mater Interfaces 11:27568–27573. https://doi.org/10.1021/acsami.9b08037
Toldrà A, Jauset-rubio M, Andree KB et al (2018) Detection and quantification of the toxic marine microalgae Karlodinium veneficum and Karlodinium armiger using recombinase polymerase amplification and enzyme-linked oligonucleotide assay. Anal Chim Acta 1039:140–148. https://doi.org/10.1016/j.aca.2018.07.057
Acknowledgements
This work has been carried out with partial financial support from Spanish Ministerio de Economía y Competitividad (SEASENSING BIO2014-56024-C2-1): “Seasensing: Microsystems for rapid, reliable and cost effective detection of toxic microalgae on-site and in real-time.” S. A-M. acknowledges support from the government of Catalonia (GENCAT) for predoctoral grant 2017FI_B2 00184, and for the training grant that allowed him to visit the University of Ioannina. The authors thank Mònica Campàs and Anna Toldrà for the provision of genomic DNA extracted from seawater samples.
Code availability
Not
Funding
This work has been carried out with partial financial support from Spanish Ministerio de Economía y Competitividad (SEASENSING BIO2014-56024-C21): “Seasensing: Microsystems for rapid, reliable and cost effective detection of toxic microalgae on-site and in real-time.”
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
AL-Madhagi, S., O’Sullivan, C.K., Prodromidis, M.I. et al. Combination of ferrocene decorated gold nanoparticles and engineered primers for the direct reagentless determination of isothermally amplified DNA. Microchim Acta 188, 117 (2021). https://doi.org/10.1007/s00604-021-04771-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04771-8