Abstract
Herein, we report a rapid and sensitive colorimetric detection of Hg2+ by designing a specific DNA probe with phosphorothioate RNA modification (PS-probe) for Hg2+ recognition and utilizing DNA-modified gold nanoparticles (DNA-AuNPs) as the transducer. The distance between two DNA-AuNPs is controlled by a linker DNA, providing the linker DNA-regulated aggregation or dispersion status of AuNPs in solution. Exonuclease III (Exo III) can trigger the recycled digestion of linker DNA strands, inhibiting the reformation of aggregated nanoparticles and hence leading to a color shift from purple to red. However, the Hg2+-induced cleavage of the PS-probe can efficiently prevent the digestion of linker DNA strands by Exo III and hence reassemble the modified AuNPs to form aggregates in purple color. Thus, a positive correlation between the linker DNA strands left and the addition of Hg2+ provides a quantitative basis for Hg2+ sensing. A linear range of A520/A700 versus Hg2+ concentration is achieved in the range 2–100 nM associated with a detection limit as low as 1.30 ± 0.04 nM. Moreover, the biosensor exhibits excellent selectivity for Hg2+. The strong selectivity behavior was confirmed by recoveries ranging from 96 to 114% in real water samples.

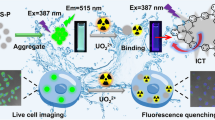
Schematic representation of sensing mechanism of Hg2+ using a DNA probe with phosphorothioate RNA modification (PS-probe) and Exo III-assisted signal amplification




Similar content being viewed by others
References
Jarup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182. https://doi.org/10.1093/bmb/ldg032
Zahir F, Rizwi SJ, Haq SK, Khan RH (2005) Low dose mercury toxicity and human health. Environ Toxicol Pharmacol 20(2):351–360. https://doi.org/10.1016/j.etap.2005.03.007
Li T, Dong S, Wang E (2009) Label-free colorimetric detection of aqueous mercury ion (Hg2+) using Hg2+-modulated G-quadruplex-based DNAzymes. Anal Chem 81(6):2144–2149. https://doi.org/10.1021/ac900188y
Nguyen VT, Kwon YS, Gu MB (2017) Aptamer-based environmental biosensors for small molecule contaminants. Curr Opin Biotechnol 45:15–23. https://doi.org/10.1016/j.copbio.2016.11.020
Kaur H, Bruno JG, Kumar A, Sharma TK (2018) Aptamers in the therapeutics and diagnostics pipelines. Theranostics 8(15):4016–4032. https://doi.org/10.7150/thno.25958
Zhang Y, Deng Y, Wang C, Li L, Xu L, Yu Y, Su X (2019) Probing and regulating the activity of cellular enzymes by using DNA tetrahedron nanostructures. Chem Sci 10(23):5959–5966. https://doi.org/10.1039/C9SC01912J
Ono A, Togashi H (2004) Highly selective oligonucleotide-based sensor for mercury(II) in aqueous solutions. Angew Chem Int Ed Engl 43(33):4300–4302. https://doi.org/10.1002/anie.200454172
Zhuang J, Fu L, Tang D, Xu M, Chen G, Yang H (2013) Target-induced structure-switching DNA hairpins for sensitive electrochemical monitoring of mercury (II). Biosens Bioelectron 39(1):315–319. https://doi.org/10.1016/j.bios.2012.07.015
Ling B, Ma Y, Chen H, Wang L (2017) A SPR aptamer sensor for mercury based on AuNPs@NaYF4:Yb,Tm,Gd upconversion luminescent nanoparticles. Anal Methods 9(42):6032–6037. https://doi.org/10.1039/C7AY01810J
Qi L, Zhao Y, Yuan H, Bai K, Zhao Y, Chen F, Dong Y, Wu Y (2012) Amplified fluorescence detection of mercury(II) ions (Hg2+) using target-induced DNAzyme cascade with catalytic and molecular beacons. Analyst 137(12):2799–2805. https://doi.org/10.1039/C2AN35437C
Song X, Wang Y, Liu S, Zhang X, Wang H, Wang J, Huang J (2018) Ultrasensitive electrochemical detection of Hg2+ based on an Hg2+-triggered exonuclease III-assisted target recycling strategy. Analyst 143(23):5771–5778. https://doi.org/10.1039/C8AN01409D
Huang PJ, van Ballegooie C, Liu J (2016) Hg2+ detection using a phosphorothioate RNA probe adsorbed on graphene oxide and a comparison with thymine-rich DNA. Analyst 141(12):3788–3793. https://doi.org/10.1039/C5AN02031J
Kiy MM, Jacobi ZE, Liu J (2012) Metal-induced specific and nonspecific oligonucleotide folding studied by FRET and related biophysical and bioanalytical implications. Chemistry 18(4):1202–1208. https://doi.org/10.1002/chem.201102515
Kiy MM, Zaki A, Menhaj AB, Samadi A, Liu J (2012) Dissecting the effect of anions on Hg2+ detection using a FRET based DNA probe. Analyst 137(15):3535–3540. https://doi.org/10.1039/C2AN35314H
Hollenstein M, Hipolito C, Lam C, Dietrich D, Perrin DM (2008) A highly selective DNAzyme sensor for mercuric ions. Angew Chem Int Ed Engl 47(23):4346–4350. https://doi.org/10.1002/anie.200800960
Zhang XB, Kong RM, Lu Y (2011) Metal ion sensors based on DNAzymes and related DNA molecules. Annu Rev Anal Chem (Palo Alto, Calif) 4:105–128. https://doi.org/10.1146/annurev.anchem.111808.073617
Liu J, Lu Y (2007) Rational design of "turn-on" allosteric DNAzyme catalytic beacons for aqueous mercury ions with ultrahigh sensitivity and selectivity. Angew Chem Int Ed Engl 46(40):7587–7590. https://doi.org/10.1002/anie.200702006
Huang PJ, Wang F, Liu J (2015) Cleavable molecular beacon for Hg2+ detection based on phosphorothioate RNA modifications. Anal Chem 87(13):6890–6895. https://doi.org/10.1021/acs.analchem.5b01362
Rosi NL, Mirkin CA (2005) Nanostructures in biodiagnostics. Chem Rev 105(4):1547–1562. https://doi.org/10.1021/cr030067f
Borghei YS, Hosseini M, Dadmehr M, Hosseinkhani S, Ganjali MR, Sheikhnejad R (2016) Visual detection of cancer cells by colorimetric aptasensor based on aggregation of gold nanoparticles induced by DNA hybridization. Anal Chim Acta 904:92–97. https://doi.org/10.1016/j.aca.2015.11.026
Yang X, Xu J, Tang X, Liu H, Tian D (2010) A novel electrochemical DNAzyme sensor for the amplified detection of Pb2+ ions. Chem Commun (Camb) 46(18):3107–3109. https://doi.org/10.1016/j.aca.2015.11.026
Zhu D, Yan Y, Lei P, Shen B, Cheng W, Ju H, Ding S (2014) A novel electrochemical sensing strategy for rapid and ultrasensitive detection of Salmonella by rolling circle amplification and DNA-AuNPs probe. Anal Chim Acta 846:44–50. https://doi.org/10.1016/j.aca.2014.07.024
Turkevich J, Stevenson PC, Hillier J (1951) A study of the nucleation and growth processes in the synthesis of colloidal gold. Discussions of the Faraday Society 11:55–75. https://doi.org/10.1039/DF9511100055
Liu J, Lu Y (2004) Accelerated color change of gold nanoparticles assembled by DNAzymes for simple and fast colorimetric Pb2+ detection. J Am Chem Soc 126(39):12298–12305. https://doi.org/10.1021/ja046628h
Wang H, Liu Y, Liu G (2018) Electrochemical biosensor using DNA embedded phosphorothioate modified RNA for mercury ion determination. ACS Sens 3(3):624–631. https://doi.org/10.1021/acssensors.7b00892
Ye BC, Yin BC (2008) Highly sensitive detection of mercury (II) ions by fluorescence polarization enhanced by gold nanoparticles. Angew Chem Int Ed 47(44):8386–8389. https://doi.org/10.1002/anie.200803069
Liu S, Leng XQ, Wang X, Pei QQ, Cui XJ, Wang Y, Huang JD (2017) Enzyme-free colorimetric assay for mercury (II) using DNA conjugated to gold nanoparticles and strand displacement amplification. Microchim Acta 7(184):1969–1976. https://doi.org/10.1007/s00604-017-2182-7
Geng Z, Zhang H, Xiong Q, Zhang Y, Zhao H, Wang G (2015) A fluorescent chitosan hydrogel detection platform for the sensitive and selective determination of trace mercury (II) in water. J Mater Chem A 3(38):19455–19460. https://doi.org/10.1039/C5TA05610A
Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA (1997) Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 277(5329):1078–1081. https://doi.org/10.1126/science.277.5329.1078
Li L, Li B, Qi Y, Jin Y (2009) Label-free aptamer-based colorimetric detection of mercury ions in aqueous media using unmodified gold nanoparticles as colorimetric probe. Anal Bioanal Chem 393(8):2051–2057. https://doi.org/10.1007/s00216-009-2640-0
Guo YM, Wang Z, Qu WS, Shao HW, Jiang XY (2011) Colorimetric detection of mercury, lead and copper ions simultaneously using protein-functionalized gold nanoparticles. Biosens Bioelectron 26(10):4064–4069. https://doi.org/10.1016/j.bios.2011.03.033
Chen YJ, Yao L, Deng Y, Pan DD, Ogabiela E, Cao JX, Adeloju SB, Chen W (2015) Rapid and ultrasensitive colorimetric detection of mercury (II) by chemically initiated aggregation of gold nanoparticles. Microchim Acta 182(13–14):2147–2154. https://doi.org/10.1007/s00604-015-1538-0
Liang GX, Wang L, Zhang HQ, Han ZX, Wu XX (2012) A colorimetric probe for the rapid and selective determination of mercury (II) based on the disassembly of gold nanorods. Microchim Acta 179:345–350. https://doi.org/10.1007/s00604-012-0882-6
Li K, Liang AH, Jiang CN, Li F, Liu QG, Jiang ZL (2012) A stable and reproducible nanosilver-aggregation-4-mercaptopyridine surface-enhanced Raman scattering probe for rapid determination of trace Hg2+. Talanta 99:890–896. https://doi.org/10.1016/j.talanta.2012.07.052
Funding
This research is supported by the Beijing Municipal Natural Science Foundation-Haidian Primitive Innovation Joint Fund Project (L182045).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 451 kb)
Rights and permissions
About this article
Cite this article
Xing, Y., Zhu, Q., Zhou, X. et al. A gold nanoparticle-based colorimetric mercury(II) biosensor using a DNA probe with phosphorothioate RNA modification and exonuclease III-assisted signal amplification. Microchim Acta 187, 214 (2020). https://doi.org/10.1007/s00604-020-4184-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-4184-0