Abstract
A new strategy has been developed for the determination of trace lead ions (Pb2+) based on hexagonal boron nitride (h-BN) laden with point defect. The defect-laden boron nitride (D-BN) was synthesized by a thermal polymerization route, in which melamine borate was used as a precursor. The defect microstructure was confirmed by photoluminescence (PL) and x-ray diffraction (XRD) techniques. As compared with h-BN, the D-BN-modified glassy carbon electrode (GCE) showed an enhanced electrochemical response towards Pb2+ peaking at − 0.551 V (vs. SCE), which was evidenced by linear sweep anodic stripping voltammetry (LSASV) results. The point defect plays a pivotal role in the electrocatalytic reaction process, which can mediate the electronic structure and surface properties of h-BN. Accordingly, the sensor presented a low detection limit of 0.15 μg/L towards Pb2+ and a wide linear response concentration range from 0.5 to 400 μg/L (correlation coefficient = 0.995). In view of its superior selectivity, stability, and reproducibility, the proposed method was applied for Pb2+ determination in real samples and exhibited satisfactory results. This work provides insight for the construction of electrochemical sensor with high-performance by engineering defects of modifying materials.
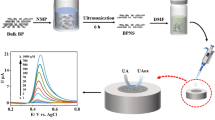
Graphical abstract

Defect-loaden h-BN exhibited enhanced electrocatalytic redox reaction towards lead ions and thus a novel Pb2+ sensor with high performances was constructed.




Similar content being viewed by others
References
Ali I, Basheer AA, Mbianda XY, Burakov A, Galunin E, Burakova I, Mkrtchyan E, Tkacher A, Grachev V (2019) Graphene based adsorbents for remediation of noxious pollutants from wastewater. Environ Int 127:160–180. https://doi.org/10.1016/j.envint.2019.03.029
Waheed A, Mansha M, Ullah N (2018) Nanomaterials-based electrochemical detection of heavy metals in water: current status, challenges and future direction. TrAC Trends Anal Chem 105:37–51. https://doi.org/10.1016/j.trac.2018.04.012
Googerdchian F, Moheb A, Emadi R, Asgari M (2018) Optimization of Pb(II) ions adsorption on nanohydroxyapatite adsorbents by applying Taguchi method. J Hazard Mater 349:186–194. https://doi.org/10.1016/j.chemosphere.2020.127560
Kang W, Pei X, Rusinek CA, Bange A, Haynes EN, Heineman WR, Papautsky I (2017) Determination of lead with a copper-based electrochemical sensor. Anal Chem 89:3345–3352. https://doi.org/10.1021/acs.analchem.6b03894
Zhao LL, Zhong SX, Fang KM, Qian ZS, Chen JR (2012) Determination of cadmium(II), cobalt(II), nickel(II), lead(II), zinc(II), and copper(II) in water samples using dual-cloud point extraction and inductively coupled plasma emission spectrometry. J Hazard Mater 239-240:206–212. https://doi.org/10.1016/j.jhazmat.2012.08.066
Vassileva E, Hoenig M (2011) Determination of the total and extractable mass fractions of cadmium and lead in mineral feed by using isotope dilution inductively coupled plasma mass spectrometry. Anal Chim Acta 701:37–44. https://doi.org/10.1016/j.aca.2011.05.045
Latif E, Arslan Z, Tyson JF (2009) Determination of lead in wine and rum samples by flow injection-hydride generation-atomic absorption spectrometry. J Hazard Mater 162:880–885. https://doi.org/10.1016/j.jhazmat.2008.05.113
Sun Q, Wang J, Tang M, Huang L, Zhang Z, Liu C, Lu X, Hunter KW, Chen G (2017) A new electrochemical system based on a flow-field shaped solid electrode and 3D-printed thin-layer flow cell: detection of Pb2+ ions by continuous flow accumulation square-wave anodic stripping voltammetry. Anal Chem 89:5024–5029. https://doi.org/10.1021/acs.analchem.7b00383
Yf S, Wang J, Li PH, Yang M, Huang XJ (2019) Highly sensitive electrochemical detection of Pb(II) based on excellent adsorption and surface Ni(II)/Ni(III) cycle of porous flower-like NiO/rGO nanocomposite. Sensors Actuators B Chem 292:136–147. https://doi.org/10.1016/j.snb.2019.04.131
Xu TT, Dai HQ, Jin YC (2020) Electrochemical sensing of lead(II) by differential pulse voltammetry using conductive polypyrrole nanoparticles. Microchim Acta 187:23. https://doi.org/10.1007/s00604-019-4027-z
Song XL, Wang Y, Liu S, Zhang X, Wang JF, Wang HW, Zhang FF, Yu JH, Huang JD (2019) A triply amplified electrochemical lead(II) sensor by using a DNAzyme and via formation of a DNA-gold nanoparticle network induced by a catalytic hairpin assembly. Microchim Acta 186:559. https://doi.org/10.1007/s00604-3612-5
Jiang Y, Cui SJ, Xia T, Sun TR, Tan HX, Yu F, Su Y, Wu SL, Wang DJ, Zhu N (2020) Real-time monitoring of heavy metals in healthcare via twistable and washable Smartsensors. Anal Chem 92:14536–14541. https://doi.org/10.1021/acs.analchem.0c02723
Gao W, Nyein HYY, Shahpar Z, Fahad HM, Chen K, Emaminejad S, Gao YJ, Tai LC, Ota H, Wu E, Bullock J, Zeng YP, Lien DH, Javey A (2016) Wearable microsensor array for multiplexed heavy metal monitoring of body fluids. ACS Sensors 1:866–874. https://doi.org/10.1021/acssensors.6b00287
Karimi-Maleh H, Cellat K, Arıkan K, Savk A, Karimi F, Ṣen F (2020) Palladium–nickel nanoparticles decorated on functionalized-MWCNT for high precision non-enzymatic glucose sensing. Mater Chem Phys 250:123024. https://doi.org/10.1016/j.matcemphys.2020.123042
Karimi-Maleh H, Arotiba OA (2020) Simultaneous determination of cholesterol, ascorbic acid and uric acid as three essential biological compounds at a carbon paste electrode modified with copper oxide decorated reduced graphene oxide nanocomposite and ionic liquid. J Colloid Surf Sci 560:208–212. https://doi.org/10.1016/j.jcis.2019.10.007
Karimi-Maleh H, Karimi F, Alizadeh M, Sanati AL (2020) Electrochemical sensors, a bright future in the fabrication of portable kits in analytical systems. Chem Rec 20:682–692. https://doi.org/10.1002/tcr.201900092
Baghayeri M, Motlagh MG, Tayebee R, Fayazi M, Narenji F (2020) Application of graphene/zinc-based metal-organic framework nanocomposite for electrochemical sensing of As (III) in water resources. Anal Chim Acta 1099:60–67. https://doi.org/10.1016/j.aca.2019.11.045
Motlagh MG, Baghayeri M (2020) Determination of trace Tl(I) by differential pulse anodic stripping voltammetry using a novel modified carbon paste electrode. J Electrochem Soc 167:066508. https://doi.org/10.1149/1945-7111/ab823cj
Nodehi M, Baghayeri M, Ansari R, Veisi H (2020) Electrochemical quantification of 17α-ethinylestradiol in biological samples using a Au/Fe3O4@TA/MWNT/GCE sensor. Mater Chem Phys 244:122687. https://doi.org/10.1016/j.matchemphys.2020.122687
Motlagh MG, Taher MA, Fayazi M, Baghayeri M, Hosseinifar A (2019) Non-enzymatic amperometric sensing of hydrogen peroxide based on vanadium pentoxide nanostructures. J Electrochem Soc 166:B367–B372. https://doi.org/10.1149/2.0521906jes
Wang YG, Zhao GH, Zhang Q, Wang H, Zhang Y, Cao W, Zhang N, Du B, Wei Q (2019) Electrochemical aptasensor based on gold modified graphene nanocomposite with different morphologies for ultrasensitive detection of Pb2+. Sensors Actuators B 288:325–331. https://doi.org/10.1016/j.snb.2019.03.010
Zhou YZ, Cui RR, Dang Y, Li Y, Zou Y (2019) Doping controlled oxygen vacancies of ZnWO4 as a novel and effective sensing platform for carbendazim and biomolecule. Sensors Actuators B 296:126680. https://doi.org/10.1016/j.snb.2019.126680
Jia Y, Jiang K, Wang HT, Yao XD (2019) The role of defect sites in nanomaterials for electrocatalytic energy conversion. Chem 5:1–27. https://doi.org/10.1016/j.chempr.2019.02.008
Zhong FL, Shi LQ, Zhao JW, Cai GH, Zheng Y, Xiao YH, Zheng Y, Jiang LL (2018) Pyrochlore Pr2Zr2-xMxO7+δ (M = Al, Ga, In) solid-state electrolytes: defect-mediated oxygen hopping pathways and enhanced NO2 sensing properties. Sensors Actuators B Chem 270:130–139. https://doi.org/10.1016/j.snb.2018.04.178
Zhang Y, Chen XS, Huang Y, Zhang C, Li F, Shu HB (2017) The role of intrinsic defects in electrocatalytic activity of monolayer VS2 basal planes for the hydrogen evolution reaction. J Phys Chem C 121:1530–1536. https://doi.org/10.1021/acs.jpcc.6b11987
Őrnek M, Wang KL, Xiang SS, Hwang C, Xie LY, Haber RA (2019) Molten salt synthesis of highly ordered and nanostructured hexagonal boron nitride. Diam Relat Mater 93:179–186. https://doi.org/10.1016/j.diamond.2019.02.010
Ma ZS, Ding HL, Liu Z, Cheng ZL (2019) Preparation and tribological properties of hydrothermally exfoliated ultrathin hexagonal boron nitride nanosheets (BNNSs) in mixed NaOH/KOH solution. J Alloys Compd 784:807–815. https://doi.org/10.1016/j.jallcom.2019.01.108
Weng QH, Wang XB, Wang X, Bando Y, Golberg D (2016) Functionalized hexagonal boron nitride nanomaterials: emerging properties and applications. Chem Soc Rev 45:3989–4012. https://doi.org/10.1039/c5cs00869g
Jung JY, Song BK, Kim YK (2019) Tunable color emission of transparent boron nitride nanophosphors towards anti-counterfeiting application. J Alloys Compd 791:81–86. https://doi.org/10.1016/j.jallcom.2019.03.269
Xiong J, Di J, Zhu WS, Li HM (2020) Hexagonal boron nitride adsorbent: synthesis, performance tailoring and applications. J Energy Chem 40:99–111. https://doi.org/10.1016/j.jechem.2019.03.002
Sajid A, Reimers JR, Ford MJ (2018) Defect states in hexagonal boron nitride: assignments of observed properties and prediction of properties relevant to quantum computation. Phys Rev B 97:064101. https://doi.org/10.1103/PhysRevB.97.064101
Shen YL, Yan H, Guo H, Long YM, Li WF (2020) Defect-rich hexagonal boron nitride for the simultaneous determination of 4-aminophenol and phenol. Sensors Actuators B Chem 303:127248. https://doi.org/10.1016/j.snb.2019.127248
Ferrari AGM, Rowley-Neale SJ, Banks CE (2020) Recent advances in 2D hexagonal boron nitride (2D-hBN) applied as the basis of electrochemical sensing platforms. Anal Bioanal Chem. https://doi.org/10.1007/s00216-020-03068-8
Zhang KL, Feng YL, Wang F, Yang ZC, Wang J (2017) Two dimensional hexagonal boron nitride (2D-hBN): synthesis, properties and applications. J Mater Chem C 5:11992–12022. https://doi.org/10.1039/c7tc04300g
Ferrari AGM, Brownson DAC, Dena ASA, Foster CW, Neale SJR, Banks CE (2020) Tailoring the electrochemical properties of 2D-hBN via physical linear defects: physicochemical, computational and electrochemical characterization. Nanoscale Adv 2:264–273. https://doi.org/10.1039/c9na00530g
Angizi S, Khalaj M, Alem SAA, Pakdel A, Willander M, Hatamie A, Simchi A (2020) Review˗towards the two-dimensional hexagonal boron nitride (2D h-BN) electrochemical sensing platforms. J Electrochem Soc 167:126513. https://doi.org/10.1149/1945-7111/abaf29
Khan AF, Brownson DAC, Randviir EP, Smith GC, Banks CE (2016) 2D hexagonal boron nitride (2D-hBN) explored for the electrochemical sensing of dopamine. Anal Chem 88:9729–9737. https://doi.org/10.1021/acs.analchem.6b02638
Chen H, Yang ZZ, Zhang ZH, Chen ZT, Chi MF, Wang S, Fu J, Dai S (2019) Construction of a nanoporous highly crystalline hexagonal boron nitride from an amorphous precursor for catalytic dehydrogenation. Angew Chem Int Ed 58:10626–10630. https://doi.org/10.1002/anie.201904996
He Y, Li DD, Gao W, Yin H, Chen F, Sun YF (2019) High-performance NO2 sensors based on spontaneously functionalized hexagonal boron nitride nanosheets via chemical exfoliation. Nanoscale 11:21909–21916. https://doi.org/10.1039/C9NR07153A
Xia D, Li H, Huang P, Mannering J, Zafa U, Baker D, Menzel R (2019) Boron-nitride/carbon-nanotube hybrid aerogels as multifunctional desulfurization agents. J Mater Chem A 7:24027–24037. https://doi.org/10.1039/C9TA06599G
Maleki M, Beitollahi A, Javadpour J, Yahya N (2015) Dual template route for the synthesis of hierarchical porous boron nitride. Ceram Int 41:3806–3813. https://doi.org/10.1016/j.ceramint.2014.11.056
Zhan YJ, Zeng YB, Li L, Guo LH, Luo F, Qiu B, Huang YJ, Lin ZY (2020) Cu2+-modified boron nitride nanosheets-supported subnanometer gold nanoparticles: an oxidase-mimicking nanoenzyme with unexpected oxidation properties. Anal Chem 92:1236–1244. https://doi.org/10.1021/acs.analchem.9b04384
Weston L, Wickramaratne D, Mackoit M, Alkauskas A, Walle CGV (2018) Native point defects and impurities in hexagonal boron nitride. Phys Rev B 97:214104. https://doi.org/10.1103/PhysRevB.97.214104
Tsushima E, Tsujimura T, Uchino T (2018) Enhancement of the deep-level emission and its chemical origin in hexagonal boron nitride. Appl Phys Lett 93:031903. https://doi.org/10.1063/1.5038168
Zhang R, Yang X, Tao Z, Wang X, Wang HX, Wang LC, Lv BL (2019) Insight into the effective aerobic oxidative cross-esterification of alcolols over Au/porous boron nitride catalyst. ACS Appl Mater Interfaces 11:46678–46687. https://doi.org/10.1021/acsami.9b14460
Dong YY, Zhang L (2018) Constructed 3D hierarchical porous wool-ball-like NiO-loaded AlOOH electrode materials for the determination of toxic metal ions. Electrochim Acta 271:27–34. https://doi.org/10.1016/j.electacta.2018.03.110
Xu L, Song XLA (2015) A novel Ti/antimony-doped tin oxide nanoparticles electrode prepared by screen printing method and its application in electrochemical degradation of C.I. Acid Red 73. Electrochim Acta 185:6–16. https://doi.org/10.1016/j.electacta.2015.10.106
Isa IM, Saidin MI, Ahmad M, Hashim N, Bakar SA, Ali NM, Si SM (2017) Chloroplatinum (II) complex-modified MWCNTs paste electrode for electrochemical determination of mercury in skin lightening cosmetics. Electrochim Acta 253:463–471. https://doi.org/10.1016/j.electacta.2017.09.092
Baghbaderani SS, Noorbakhsh A (2019) Novel chitosan-Nafion composite for fabrication of highly sensitive impedimetric and colorimetric As(III) aptasensor. Biosens Bioelectron 131:1–8. https://doi.org/10.1016/j.bios.2019.01.059
Zhao G, Liu G (2019) Synthesis of a three-dimensional (BiO)2CO3@single-walled carbon nanotube nanocomposite and its application for ultrasensitive detection of trace Pb(II) and Cd(II) by incorporating Nafion. Sensors Actuators B Chem 288:71–79. https://doi.org/10.1016/j.snb.2019.02.106
Karthikeyan R, Nelson DJ, Ajith A, John SA (2019) Hetero atoms doped carbon dots modified electrodes for the sensitive and selective determination of phenolic anti-oxidant in coconut oil. J Electroanal Chem 848:113297. https://doi.org/10.1016/j.jelechem.2019.113297
Kumar SP, Giribabu K, Manigandan R, Munusamy S, Muthanmizh S, Padmanaban A, Dhanasekaran T, Suresh R, Narayanan V (2016) Simultaneous determination of paracetamol and 4-aminophenol based on poly(chromium Schiff base complex) modified electrode at nanomolar levels. Electrochim Acta 194:116–126. https://doi.org/10.1016/j.electacta.2016.02.087
Gan T, Wang ZK, Gao JY, Sun JY, Wu KB, Wang HB, Liu YM (2019) Morphology–dependent electrochemical activity of Cu2O polyhedrons and construction of sensor for simultaneous determination of phenolic compounds with graphene oxide as reinforcement. Sensors Actuators B Chem 282:549–558. https://doi.org/10.1016/j.snb.2018.11.102
Lu ZW, Lin XN, Zhang JJ, Dai WL, Liu BC, Mo GQ, Ye JP, Ye JS (2019) Ionic liquid/poly-l-cysteine composite deposited on flexible and hierarchical porous laser-engraved graphene electrode for high-performance electrochemical analysis of lead ion. Electrochim Acta 295:514–523. https://doi.org/10.1016/j.electacta.2018.10.176
Ma Y, Wang YC, Xie DH, Gu Y, Zhu XL, Zhang HM, Wang GZ, Zhang YX, Zhao HJ (2018) Hierarchical MgFe-layered double hydroxide microsphere/grapheme composite for simultaneous electrochemical determination of trace Pb(II) and Cd(II). Chem Eng J 347:953–962. https://doi.org/10.1016/j.cej.2018.04.172
Shi L, Li Y, Rong X, Wang Y, Ding S (2017) Facile fabrication of a novel 3D grapheme framework/Bi nanoparticle film for ultrasensitive electrochemical assays of heavy metal ions. Anal Chim Acta 968:21–29. https://doi.org/10.1016/j.aca.2017.03.013
Funding
This work is supported by the National Natural Science Foundation of China (21005053), the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the Project of Scientific and Technologic Infrastructure of Suzhou (SZS201708).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 2197 kb)
Rights and permissions
About this article
Cite this article
Shen, Y., Ouyang, H., Li, W. et al. Defect-enhanced electrochemical property of h-BN for Pb2+ detection. Microchim Acta 188, 40 (2021). https://doi.org/10.1007/s00604-020-04691-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04691-z