Abstract
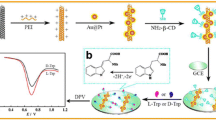
A novel chiral sensing platform, 6-O-α-maltosyl-β-cyclodextrin (Mal-βCD)-based film, is proposed for selective electrochemical recognition of tyrosine (Tyr) enantiomers. Black phosphorus nanosheets (BP NSs) and Mal-βCD modified glassy carbon electrode (Mal-βCD/BP NSs/GCE) were prepared by a layer-to-layer drop-casting method, and the platform was easy to fabricate and facile to operate. It is proposed that the amino and hydroxyl groups of the Tyr enantiomers and the chiral hydroxyl groups of Mal-βCD selectively form intermolecular hydrogen bonds to dominate effective chiral recognition. Two linear equations of Ip (μA) = 11.40 CL-Tyr (mM) + 0.28 (R2 = 0.99147) and Ip (μA) = 7.96 CD-Tyr (mM) + 0.22 (R2 = 0.99583) in the concentration range 0.01–1.00 mM have been obtained. The limits of detection (S/N=3) for L-Tyr and D-Tyr were 4.81 and 6.89 µM, respectively. An interesting phenomenon was that the value of IL-Tyr/ID-Tyr (1.51) in this work was slightly higher than the value of IL-Trp/ID-Trp (1.49) reported in our previous study, where tryptophan (Trp) enantiomers were electrochemically recognized by Nafion (NF)-stabilized BPNSs-G2-β-CD composite. The two similar sensors fabricated by different methods showed different recognition ability toward either Tyr or Trp enantiomers, and the underlying mechanism was discussed in detail. More importantly, the proposed chiral sensor enables prediction of the percentages of D-Tyr in racemic Tyr mixtures. The chiral sensor may provide a novel approach for the fabrication of novel chiral platforms in the practical detection of L- or D-enantiomer in racemic Tyr mixtures.
Graphical abstract









Similar content being viewed by others
References
Biedermann F, Nau WM (2014) Noncovalent chirality sensing ensembles for the detection and reaction monitoring of amino acids, peptides, proteins, and aromatic drugs. Angew Chem Int Ed 53(22):5694–5699
Di Pietrantonio F, Benetti M, Cannata D, Verona E, Girasole M, Fosca M et al (2016) A shear horizontal surface acoustic wave biosensor for a rapid and specific detection of D-serine. Sensors Actuators B Chem 226:1–6
Jin GP, Lin XQ (2004) The electrochemical behavior and amperometric determination of tyrosine and tryptophan at a glassy carbon electrode modified with butyrylcholine. Electrochem Commun 6(7):742
Xing SF, Sun XF, Taylor AA, Walker SL, Wang YF, Wang SG (2015) D-amino acids inhibit initial bacterial adhesion: thermodynamic evidence. Biotechnol Bioeng 112(4):696–704
Konya Y, Taniguchi M, Furuno M, Nakano Y, Tanaka N, Fukusaki E (2018) Mechanistic study on the high-selectivity enantioseparation of amino acids using a chiral crown ether-bonded stationary phase and acidic, highly organic mobile phase by liquid chromatography/time-of-flight mass spectrometry. J Chromatogr A 1578:35–44
Zhang JD, Kabir KMM, Donald WA (2018) Metal-ion free chiral analysis of amino acids as small as proline using high-definition differential ion mobility mass spectrometry. Anal Chim Acta 1036:172–178
Jafari M, Tashkhourian J, Absalan G (2018) Chiral recognition of tryptophan enantiomers using chitosan-capped silver nanoparticles: scanometry and spectrophotometry approaches. Talanta 178:870–878
Nian S, Pu L (2017) Amphiphilic polymer-based fluorescent probe for enantioselective recognition of amino acids in immiscible water and organic phases. Chemistry 23(71):18066–18073
Nie R, Bo X, Wang H, Zeng L, Guo L (2013) Chiral electrochemical sensing for tyrosine enantiomers on glassy carbon electrode modified with cysteic acid. Electrochem Commun 27:112–115
Bao L, Dai J, Yang L, Ma J, Tao Y, Deng L et al (2018) Electrochemical recognition of tyrosine enantiomers based on chiral ligand exchange with sodium alginate as the chiral selector. J Electrochem Soc 167:486–491
Shi X, Wang Y, Peng C, Zhang Z, Chen J, Zhou X, Jiang H (2017) Enantiorecognition of tyrosine based on a novel magnetic electrochemical chiral sensor. Electrochim Acta 241:386–394
Dong S, Bi Q, Qiao C, Sun Y, Zhang X, Lu X, Zhao L (2017) Electrochemical sensor for discrimination tyrosine enantiomers using graphene quantum dots and beta-cyclodextrins composites. Talanta 173:94–100
Zor E, Morales-Narvaez E, Alpaydin S, Bingol H, Ersoz M, Merkoci A (2017) Graphene-based hybrid for enantioselective sensing applications. Biosens Bioelectron 87:410–416
Zou J, Yu JG (2019) Chiral recognition of tyrosine enantiomers on a novel bis-aminosaccharides composite modified glassy carbon electrode. Anal Chim Acta 1088:35–44
Zou J, Chen XQ, Zhao GQ, Jiang XY, Jiao FP, Yu JG (2019) A novel electrochemical chiral interface based on the synergistic effect of polysaccharides for the recognition of tyrosine enantiomers. Talanta 195:628–637
Tao Y, Chu F, Gu X, Kong Y, Lv Y, Deng L (2018) A novel electrochemical chiral sensor for tyrosine isomers based on a coordination-driven self-assembly. Sensors Actuators B Chem 255:255–261
Niu X, Mo Z, Yang X, Shuai C, Liu N, Guo R (2019) Graphene-ferrocene functionalized cyclodextrin composite with high electrochemical recognition capability for phenylalanine enantiomers. Bioelectrochemistry 128:74–82
Niu X, Mo Z, Yang X, Sun M, Zhao P, Li Z, Ouyang M, Liu Z, Gao H, Guo R, Liu N (2018) Advances in the use of functional composites of beta-cyclodextrin in electrochemical sensors. Mikrochim Acta 185(7):328
Wu D, Tan W, Yu Y, Yang B, Li H, Kong Y (2018) A facile avenue to prepare chiral graphene sheets as electrode modification for electrochemical enantiorecognition. Anal Chim Acta 1033:58–64
Yang X, Niu X, Mo Z, Guo R, Liu N, Zhao P, Liu Z (2019) Electrochemical chiral interface based on the Michael addition/Schiff base reaction of polydopamine functionalized reduced graphene oxide. Electrochim Acta 319:705–715
Zhou J, Chen Q, Wang Y, Han Q, Fu Y (2012) Stereoselectivity of tyrosine enantiomers in electrochemical redox reactions on gold matrices. Electrochim Acta 59:45–48
Xu J, Wang Q, Xuan C, Xia Q, Lin X, Fu Y (2016) Chiral recognition of tryptophan enantiomers based on β-cyclodextrin-platinum nanoparticles/graphene nanohybrids modified electrode. Electroanal 28:868–873
Qian J, Yi Y, Zhang D, Zhu G (2019) Electrochemical recognition of tryptophan enantiomers using a multi-walled carbon nanotube@polydopamine composite loaded with copper(II). Mikrochim Acta 186(6):358
Liang HJ, Ling TR, Rick JF, Chou TC (2005) Molecularly imprinted electrochemical sensor able to enantroselectively recognize d and l-tyrosine. Anal Chim Acta 542(1):83–89
Ye Q, Yin ZZ, Wu H, Wu D, Tao Y, Kong Y (2020) Decoration of glutathione with copper-platinum nanoparticles for chirality sensing of tyrosine enantiomers. Electrochem Commun 110:106638
Cho SY, Lee Y, Koh HJ, Jung H, Kim JS, Yoo HW, Kim J, Jung HT (2016) Superior chemical sensing performance of black phosphorus: comparison with MoS2 and graphene. Adv Mater 28(32):7020–7028
Cai J, Gou X, Sun B, Li W, Li D, Liu J, Hu F, Li Y (2019) Porous graphene-black phosphorus nanocomposite modified electrode for detection of leptin. Biosens Bioelectron 137:88–95
Chen W, Ouyang J, Yi X, Xu Y, Niu C, Zhang W, Wang L, Sheng J, Deng L, Liu YN, Guo S (2018) Black phosphorus nanosheets as a neuroprotective nanomedicine for neurodegenerative disorder therapy. Adv Mater 30(3):1703458
Zou J, Yu JG (2020) Nafion-stabilized black phosphorus nanosheets-maltosyl-beta-cyclodextrin as a chiral sensor for tryptophan enantiomers. Mater Sci Eng C Mater Biol Appl 112:110910
Xiao Q, Lu S, Huang C, Su W, Zhou S, Sheng J, Huang S (2017) An electrochemical chiral sensor based on amino-functionalized graphene quantum dots/β-cyclodextrin modified glassy carbon electrode for enantioselective detection of tryptophan isomers. J Iran Chem Soc 14(9):1957–1970
Gao LF, Xu JY, Zhu ZY, Hu CX, Zhang L, Wang Q, Zhang HL (2016) Small molecule-assisted fabrication of black phosphorus quantum dots with a broadband nonlinear optical response. Nanoscale 8(33):15132–15136
Wang J, Li M, Shi Z, Li N, Gu Z (2002) Direct electrochemistry of cytochrome c at a glassy carbon electrode modified with single-wall carbon nanotubes. Anal Chem 74:1993–1997
Alam AU, Qin Y, Howlader MMR, Hu NX, Deen MJ (2018) Electrochemical sensing of acetaminophen using multi-walled carbon nanotube and β-cyclodextrin. Sensors Actuators B Chem 254:896–909
Huang JY, Zhao L, Lei W, Wen W, Wang YJ, Bao T, Xiong HY, Zhang XH, Wang SF (2018) A high-sensitivity electrochemical aptasensor of carcinoembryonic antigen based on graphene quantum dots-ionic liquid-Nafion nanomatrix and DNAzyme-assisted signal amplification strategy. Biosens Bioelectron 99:28–33
Ogston AG (1948) Interpretation of experiments on metabolic processes, using isotopic tracer elements. Nature 162:963
Douhal A (2004) Ultrafast guest dynamics in cyclodextrin nanocavities. Chem Rev 104:1955–1976
Acknowledgments
The authors are grateful for the financial support from the National Natural Science Foundation of China (Grant No. 51674292), the Provincial Natural Science Foundation of Hunan (Grant No. 2016JJ1023), Key Area Research and Development Program of Guangdong Province, China (No. 2020B090919001), Hunan Graduate Education Innovation and Professional Ability Improvement Project (Grant No. CX20200329), and the Fundamental Research Funds for the Central Universities of Central South University (Grant No. 2020zzts056).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 389 kb)
Rights and permissions
About this article
Cite this article
Zou, J., Lan, XW., Zhao, GQ. et al. Immobilization of 6-O-α-maltosyl-β-cyclodextrin on the surface of black phosphorus nanosheets for selective chiral recognition of tyrosine enantiomers. Microchim Acta 187, 636 (2020). https://doi.org/10.1007/s00604-020-04606-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04606-y