Abstract
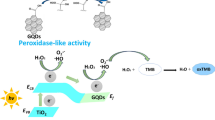
Hemin as the organic linker ligand and Cu (II) as the metal center were applied to prepare a copper-metal-organic framework (Cu-hemin-MOF) via one-step hydrothermal method. Characterization using scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FT-IR), X-ray photoelectron spectroscopy (XPS), and X-ray powder diffraction (XRD) demonstrate that the acquired Cu-hemin-MOF possesses the appearance of 3D ball-flower shape with the existence of C, N, O, Fe, and Cu on the surface. Further study found that this 3D ball-flower shaped Cu-hemin-MOF exhibits peroxidase-like activity, which can catalyze the peroxidase substrate of o-phenylenediamine (OPD) to generate 2,3-diaminophenazine (DAP) in the presence of H2O2. DAP has a yellow color and also emits a strong fluorescence when excited by ultraviolet light. Interestingly, Cu-hemin-MOF’s peroxidase-like activity can be strongly inhibited by glutathione (GSH). In view of this, a dual readout (fluorescence and colorimetry) was proposed to detect GSH for the first time. Under optimal conditions, the proposed method exhibits good linear relationship between the signal response (fluorescence and colorimetry) and the concentration of GSH, and low limits of detection (LOD) of 2.3 and 26.6 nM, respectively.
Graphical abstract






Similar content being viewed by others
References
Yang XF, Huang Q, Zhong Y, Li Z, Li H, Lowry M, Escobedo JO, Strongin RM (2014) A dual emission fluorescent probe enables simultaneous detection of glutathione and cysteine/homocysteine. Chem Sci 5:2177–2183. https://doi.org/10.1039/C4SC00308J
Gao X, Li X, Li L, Zhou J, Ma H (2015) A simple fluorescent offon probe for the discrimination of cysteine from glutathione. Chem Commun 51:9388–9390. https://doi.org/10.1039/c5cc02788h
Xu Y, Li RX, Zhou XJ, Li WQ, Ernest U, Wan H, Li L, Chen HY, Yuan ZW (2019) A visible and near-infrared, dual emission fluorescent probe based on thiol reactivity for selectively tracking mitochondrial glutathione in vitro. Talanta 205:120125. https://doi.org/10.1016/j.talanta.2019.120125
Mandal PK, Saharan S, Tripathi M, Murari G (2015) Brain glutathione levels-a novel biomarker for mild cognitive impairment and Alzheimer's disease. Biol Psychiatry 78:702–710. https://doi.org/10.1016/j.biopsych.2015.04.005
Braidy N, Zarka M, Welch J, Bridge W (2015) Therapeutic approaches to modulating glutathione levels as a pharmacological strategy in Alzheimer's disease. Curr Alzheimer Res 12:298–313. https://doi.org/10.2174/1567205012666150302160308
Lu SC, Mato JM, Espinosa-Diez C, Lamas S (2016) MicroRNA-mediated regulation of glutathione and methionine metabolism and its relevance for liver disease. Free Radic Biol Med 100:66–72. https://doi.org/10.1016/j.freeradbiomed.2016.03.021
Sun Y, Zheng Y, Wang C, Liu Y (2018) Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells article. Cell Death Dis 9:753. https://doi.org/10.1038/s41419-018-0794-4
Ngamchuea K, Batchelor-McAuley C, Williams C, Godlewska BR, Sharpley AL, Cowen PJ, Compton RG (2018) Salivary glutathione in bipolar disorder: a pilot study. J Affect Disord 238:277–280. https://doi.org/10.1016/j.jad.2018.05.041
Townsend DM, Tew KD, Tapiero H (2003) The importance of glutathione in human disease. Biomed Pharmacother 57:145–155. https://doi.org/10.1016/s0753-3322(03)00043-x
Benhar M, Shytaj IL, Stamler JS, Savarino A (2016) Dual targeting of the thioredoxin and glutathione systems in cancer and HIV. J Clin Invest 126:1630–1639. https://doi.org/10.1172/JCI85339
Bhaskar A, Munshi M, Khan SZ, Fatima S, Arya R, Jameel S, Singh A (2015) Measuring glutathione redox potential of HIV-1-infected macrophages. J Biol Chem 290:1020–1038. https://doi.org/10.1074/jbc.M114.588913
Sun XN, Heinrich P, Berger RS, Oefner PJ, Dettmer K (2019) Quantification and 13C-tracer analysis of total reduced glutathione by HPLC-QTOFMS/MS. Anal Chim Acta 1080:127–137. https://doi.org/10.1016/j.aca.2019.07.001
Fahrenholz T, Wolle MM, Kingston HM, Faber S, Kernnd JC, Pamuku M, Miller L, Chatragadda H, Kogelnik A (2015) Molecular speciated isotope dilution mass spectrometric methods for accurate, reproducible and direct quantification of reduced, oxidized and total glutathione in biological samples. Anal Chem 87:1232–1240. https://doi.org/10.1021/ac503933t
Stobiecka M, Hepel M (2010) Rapid functionalization of metal nanoparticles by moderator–tunable ligand–exchange process for biosensor designs. Sensors Actuators B Chem 149:373–380. https://doi.org/10.1016/j.snb.2010.06.049
Peng H, Jian M, Huang Z, Wang W, Deng H (2018) Facile electrochemiluminescence sensing platform based on high-quantum-yield gold nanocluster probe for ultrasensitive glutathione detection. Biosens Bioelectron 105:71–76. https://doi.org/10.1016/j.bios.2018.01.021
Lei P, Zhou Y, Zhu RQ, Liu Y, Dong C, Shuang SM (2019) Facile synthesis of iron phthalocyanine functionalized N,B–doped reduced graphene oxide nanocomposites and sensitive electrochemical detection for glutathione. Sensors Actuators B Chem 297:126756. https://doi.org/10.1016/j.snb.2019.126756
Zhu WY, Jiang GY, Xu L, Li BZ, Cai QZ, Jiang HJ, Zhou XM (2015) Facile and controllable one-step fabrication of molecularly imprinted polymer membrane by magnetic field directed self-assembly for electrochemical sensing of glutathione. Anal Chim Acta 886:37–47. https://doi.org/10.1016/j.aca.2015.05.036
Jiao L, Wang Y, Jiang HL, Xu Q (2018) Metal-organic frameworks as platforms for catalytic applications. Adv Mater 30:1703663. https://doi.org/10.1002/adma.201703663
Xu Y, Li Q, Xue H, Pang H (2018) Metal-organic frameworks for direct electrochemical applications. Coord Chem Rev 376:292–318. https://doi.org/10.1016/j.ccr.2018.08.010
Zhang XL, Li GL, Wu D, Li XL, Hu N, Chen J, Chen G, Wu YN (2019) Recent progress in the design fabrication of metal-organic frameworks-based nanozymes and their applications to sensing and cancer therapy. Biosens Bioelectron 137:178–198. https://doi.org/10.1016/j.bios.2019.04.061
Xu M, Yang SS, Gu ZY (2018) Frontispiece: two-dimensional metal-organic framework nanosheets: a rapidly growing class of versatile Nanomaterials for gas separation, MALDI-TOF matrix and biomimetic applications. Chem Eur J 24:15131–15142. https://doi.org/10.1002/chem.201885761
Gkaniatsou E, Sicard C, Ricoux R, Benahmed L, Bourdreux F, Zhang Q, Serre C, Mahy JP, Steunou N (2018) Enzyme encapsulation in mesoporous metal–organic frameworks for selective biodegradation of harmful dye molecules. Angew Chem Int Ed Eng 57:16141–16146. https://doi.org/10.1002/anie.201811327
An HD, Li MM, Gao J, Zhang ZJ, Ma SQ, Chen Y (2019) Incorporation of biomolecules in metal-organic frameworks for advanced applications. Coord Chem Rev 384:90–106. https://doi.org/10.1016/j.ccr.2019.01.001
Huang WY, Liu N, Zhang XD, Wu MH, Tang L (2017) Metal organic framework g-C3N4/MIL-53(Fe) heterojunctions with enhanced photocatalytic activity for Cr (VI) reduction under visible light. Appl Surf Sci 425:107–116. https://doi.org/10.1016/j.apsusc.2017.07.050
Wang Y, Zhu Y, Binyam A, Liu M, Wu Y, Li F (2016) Discovering the enzyme mimetic activity of metal-organic framework (MOF) for label-free and colorimetric sensing of biomolecules. Biosens Bioelectron 86:432–438. https://doi.org/10.1016/j.bios.2016.06.036
Yu L, Chen HX, Yue J, Chen XF, Sun MT, Hou J, Alamry KA, Marwani HM, Wang XK, Wang SH (2020) Europium metal-organic framework for selective and sensitive detection of doxycycline based on fluorescence enhancement. Talanta 207:120297. https://doi.org/10.1016/j.talanta.2019.120297
Zhao WW, Peng JL, Wang WK, Liu SJ, Zhao Q, Huang W (2019) Ultrathin films of a metal-organic framework prepared from 2-methylimidazole, manganese (II) and cobalt (II) with strong oxidase-mimicking activity for colorimetric determination of glutathione and glutathione reductase activity. Microchim Acta 186:340–363. https://doi.org/10.1016/j.ccr.2018.08.023
Chen J, Yu C, Zhao YL, Niu YZ, Zhang L, Yu YJ, Wu J, He JL (2017) A novel non-invasive detection method for the FGFR3 gene mutation in maternal plasma for a fetal achondroplasia diagnosis based on signal amplification by hemin-MOFs/PtNPs. Biosens Bioelectron 91:892–899. https://doi.org/10.1016/j.bios.2016.10.067
Alizadeh N, Salimi A, Hallaj R, Fathi F, Soleimani F (2018) Ni-hemin metal–organic framework with highly efficient peroxidase catalytic activity: toward colorimetric cancer cell detection and targeted therapeutics. J Nanobiotechnol 16:93. https://doi.org/10.1186/s12951-018-0421-7
Choi HS, Yang XG, Liu GC, Kim DS, Yang JH, Lee JH, Han SO, Lee J, Kim SW (2020) Development of co-hemin MOF/chitosan composite based biosensor for rapid detection of lactose. J Taiwan Inst Chem Eng 00:1–7. https://doi.org/10.1016/j.jtice.2020.07.021
Wang L, Yang H, He J, Zhang YY, Yu J, Song YH (2016) Cu-Hemin metal-organic-frameworks/chitosan-reduced graphene oxide nanocomposites with peroxidase-like bioactivity for electrochemical sensing. Electrochim Acta 213:691–697. https://doi.org/10.1016/j.electacta.2016.07.162
Song YG, Shan BX, Feng BW, Xu PF, Zeng Q, Su D (2018) A novel biosensor based on ball-flower-like cu-hemin MOF grown on elastic carbon foam for trichlorfon detection. RSC Adv 8:27008–27015. https://doi.org/10.1039/C8RA04596H
He J, Yang H, Zhang YY, Yu J, Miao LF, Song YH, Wang L (2016) Smart nanocomposites of Cu-nemin metal-organic frameworks for electrochemical glucose biosensing. Sci Rep 6:36637. https://doi.org/10.1038/srep36637
Nagababu E (2016) Ferriheme catalyzes nitric oxide reaction with glutathione to form Snitrosoglutathione: a novel mechanism for formation of S-nitrosothiols. Free Radic Biol Med 101:296–304. https://doi.org/10.1016/j.freeradbiomed.2016.09.015
Liu FF, He J, Zeng ML, Hao J, Guo QH, Song YH, Wang L (2016) Cu–hemin metal-organic frameworks with peroxidase-like activity as peroxidase mimics for colorimetric sensing of glucose. J Nanopart Res 18:106. https://doi.org/10.1007/s11051-016-3416-z
Xie SB, Ye JW, Yuan YL, Chai YQ, Yuan R (2015) A multifunctional hemin@metal–organic framework and its application to construct an electrochemical aptasensor for thrombin detection. Nanoscale 7:18232–18238. https://doi.org/10.1039/c5nr04532k
Carné A, Carbonell C, Imaz I, Maspoch D (2011) Nanoscale metal-organic materials. Chem Soc Rev 40:291–305. https://doi.org/10.1039/c0cs00042f
Taylor-Pashow KML, Rocca JD, Xie ZG, Tran S, Lin WB (2009) Postsynthetic modifications of Iron-carboxylate nanoscale metal-organic frameworks for imaging and drug delivery. J Am Chem Soc 131:14261–14263. https://doi.org/10.1021/ja906198y
Areias MCC, Shimizu K, Compton RG (2016) Voltammetric detection of glutathione: an adsorptive stripping voltammetry approach. Analyst 141:2904–2910. https://doi.org/10.1039/C6AN00550K
Hanko M, Svorc L, Plankova A, Mikus P (2019) Overview and recent advances in electrochemical sensing of glutathione-a review. Anal Chim Acta 1062:1–27. https://doi.org/10.1016/j.aca.2019.02.052
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 81772290 and 81271930]; Graduate Scientific Research and Innovation Foundation of Chongqing, China [grant number CYB19041],;Fundamental Research Funds for the Central Universities [grant numbers 2019CDYGZD007 and 2020CDCGSW052]; Brew Microorganisms Technology and Application of Key Laboratory Project in Sichuan Province [grant number, NJ2018-01]; Chongqing science and technology commission [grant number CSTC2018jcyjAX0062]; Strong-Flavor Baijiu Solid-State Fermentation Key Laboratory of China light industry [grant number 2019JJ002]; Chongqing Graduate Tutor Team Construction Project, Analytical, and Testing Center of Chongqing University for SEM, FT-IR, XRD, and XPS; and the sharing fund of Chongqing University’s large equipment for financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 848 kb).
Rights and permissions
About this article
Cite this article
Chen, X., Wang, X., Cao, G. et al. Colorimetric and fluorescent dual-identification of glutathione based on its inhibition on the 3D ball-flower shaped Cu-hemin-MOF’s peroxidase-like activity. Microchim Acta 187, 601 (2020). https://doi.org/10.1007/s00604-020-04565-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04565-4