Abstract
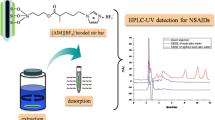
A thin-film based on 3-aminopropyl triethoxysilane surface-modified Ce-doped zinc–aluminum layered double hydroxide was synthesized on the inner surface of an aluminum tube. It has been applied to in-tube stir bar sorptive extraction of nonsteroidal anti-inflammatory drugs in saliva samples followed by high-performance liquid chromatography. The sorbent was characterized by scanning electron microscopy, X-ray diffraction, Fourier-transform infrared spectroscopy, energy-dispersive X-ray spectroscopy, and elemental mapping. The extraction parameters including sample pH (4.2), extraction time (10 min), stirring speed (800 rpm), type of eluent (acidified tetrahydrofuran), eluent volume (100 μL), and desorption time (6 min) were thoroughly optimized. Under the optimum conditions, limits of detection were found to be less than 5.0 ng mL−1. Calibration plots were linear within the range 10–1000 ng mL−1 (R2 > 0.9982). Absolute recoveries were calculated in the range 63.5 to 72.4%. The repeatability (intra- and inter-day precision) and reproducibility (tube-to-tube precision) at concentrations of 50, 250, and 500 ng mL−1 were less than 7.6% and 9.4%, respectively. The method accuracy based on the relative error was calculated at these concentrations and ranged from − 4.9 to − 9.3% for intra-day relative error (%) and − 6.8 to − 11% for inter-day relative error (%). Finally, the method applicability was examined for the determination of nonsteroidal anti-inflammatory drugs in saliva samples, and good relative recoveries were obtained within the range 86.5 to 95.2%. As a result, the introduced method can be applied as a suitable alternative to measuring nonsteroidal anti-inflammatory drugs in biological fluids.

A surface-modified Ce-doped ZnAl LDH thin film was synthesized on the inner surface of an Al tube and applied for in-tube stir bar sorptive extraction of NSAIDs in saliva.




Similar content being viewed by others
References
Prasad S, Tyagi AK, Aggarwal BB (2016) Detection of inflammatory biomarkers in saliva and urine: potential in diagnosis, prevention, and treatment for chronic diseases. Exp Biol Med 241:783–799
Seidi S, Rezazadeh M, Alizadeh R (2019) Miniaturized sample preparation methods for saliva analysis. Bioanalysis 11:119–148
Kepekci Tekkeli ŞE, Durmus Z (2019) Magnetic solid phase extraction applications combined with analytical methods for determination of drugs in different matrices review. J Chil Chem Soc 64:4448–4458
Mao X, He M, Chen B, Hu B (2016) Membrane protected C18 coated stir bar sorptive extraction combined with high performance liquid chromatography–ultraviolet detection for the determination of non-steroidal anti–inflammatory drugs in water samples. J Chromatogr A 1472:27–34
Soares da Silva Burato J, Vargas Medina DA, de Toffoli AL et al (2020) Recent advances and trends in miniaturized sample preparation techniques. J Sep Sci 43:202–225
David F, Ochiai N, Sandra P (2019) Two decades of stir bar sorptive extraction: a retrospective and future outlook. TrAC trends Anal Chem 112:102–111
Sánchez-Rojas F, Bosch-Ojeda C, Cano-Pavón JM (2009) A review of stir bar sorptive extraction. Chromatographia 69:79–94
David F, Sandra P (2007) Stir bar sorptive extraction for trace analysis. J Chromatogr A 1152:54–69
Kawaguchi M, Ito R, Saito K, Nakazawa H (2006) Novel stir bar sorptive extraction methods for environmental and biomedical analysis. J Pharm Biomed Anal 40:500–508
Farhadi K, Firuzi M, Hatami M (2015) Stir bar sorptive extraction of propranolol from plasma samples using a steel pin coated with a polyaniline and multiwall carbon nanotube composite. Microchim Acta 182:323–330
Dong N, Zhang L, Yao J, Ma P, He J, Li T, Wang Y (2019) Monohydroxycucurbit[7]uril–coated stir–bar sorptive extraction coupled with high–performance liquid chromatography for the determination of apolar and polar organic compounds. Microchim Acta 186:846
Camino-Sánchez FJ, Rodríguez-Gómez R, Zafra-Gómez A et al (2014) Stir bar sorptive extraction: recent applications, limitations and future trends. Talanta 130:388–399
Bader N (2018) Stir bar sorptive extraction as a sample preparation technique for chromatographic analysis: an overview. Asian J Nanosci Mater 1:56–62
Piryaei M (2018) Layered double hydroxide films on nanoporous anodic aluminum oxide/aluminum wire: a new fiber for rapid analysis of Origanum vulgare essential oils. Nat Prod Res 32:243–245
Sajid M, Basheer C (2016) Layered double hydroxides: emerging sorbent materials for analytical extractions. TrAC Trends Anal Chem 75:174–182
Tian R, Liang R, Wei M, et al (2016) Applications of layered double hydroxide materials: recent advances and perspective. In: Struct Bond 172:65–84
Tang S, Yao Y, Chen T et al (2020) Recent advances in the application of layered double hydroxides in analytical chemistry: a review. Anal Chim Acta 1103:32–48
Aghaziarati M, Yamini Y, Shamsayei M (2020) An electrodeposited terephthalic acid–layered double hydroxide (Cu–Cr) nanosheet coating for in–tube solid–phase microextraction of phthalate esters. Microchim Acta 187:118
Li X, Lan H, Hartonen K, Jussila M, Wang X, Riekkola ML (2020) Layered double hydroxide/poly(vinylpyrrolidone) coated solid phase microextraction arrow for the determination of volatile organic compounds in water. J Sep Sci. https://doi.org/10.1002/jssc.202000239
Sajid M, Basheer C, Daud M, Alsharaa A (2017) Evaluation of layered double hydroxide/graphene hybrid as a sorbent in membrane-protected stir–bar supported micro–solid–phase extraction for determination of organochlorine pesticides in urine samples. J Chromatogr A 1489:1–8
Dinari M, Neamati S (2020) Surface modified layered double hydroxide/polyaniline nanocomposites: synthesis, characterization and Pb2+ removal. Colloids Surfaces A Physicochem Eng Asp 589:124438
Adlnasab L, Shahdousti P, Ahmar H (2020) Layered double hydroxide intercalated with tyrosine for ultrasonic-assisted microextraction of tramadol and methadone from biological samples followed by GC/MS analysis. Microchim Acta 187:1–11
Manouchehri M, Seidi S, Rouhollahi A, Noormohammadi H, Shanehsaz M (2020) Micro solid phase extraction of parabens from breast milk samples using Mg-Al layered double hydroxide functionalized partially reduced graphene oxide nanocomposite. Food Chem 314:126223
Seidi S, Doroudian M (2020) Electrospun NiFe layered double hydroxide/nylon 6 composite nanofibers as a sorbent for micro solid phase extraction by packed sorbent of non-steroidal anti-inflammatory drugs in human blood. J Chromatogr A 1614:460718
Leite VDSA, Constantino VRL, Izumi CMS et al (2020) A dispersive solid phase extraction–based method for chromium(VI) analysis using a Zn–Al layered double hydroxide intercalated with l–aspartic acid as a dissolvable adsorbent. New J Chem 44:10087–10094
Yuan Z, Zhou W, Chen Z (2020) Flower–like layered double hydroxide-modified stainless–steel fibers for online in–tube solid–phase microextraction of Sudan dyes. J Sep Sci 43:1316–1322
Ghani M (2019) In–situ growth of zinc-aluminum-layered double hydroxide on nanoporous anodized aluminum bar for stir-bar sorptive extraction of phenolic acids. Microchem J 147:1173–1179
Alipour F, Raoof JB, Ghani M (2020) In–situ synthesis of flower like Co3O4 nanorod arrays on anodized aluminum substrate templated from layered double hydroxide as a nanosorbent for thin film microextraction of acidic drugs followed by HPLC–UV quantitation. J Chromatogr B 1144:122090
Zhou W, Wang X, Wang C et al (2020) Surface area expansion by flower–like nanoscale layered double hydroxides for high efficient stir bar sorptive extraction. Anal Chim Acta 1116:45–52
Costa FR, Leuteritz A, Wagenknecht U et al (2008) Intercalation of Mg–Al layered double hydroxide by anionic surfactants: preparation and characterization. Appl Clay Sci 38:153–164
Park AY, Kwon H, Woo AJ, Kim SJ (2005) Layered double hydroxide surface modified with (3–aminopropyl) triethoxysilane by covalent bonding. Adv mater 17:106–109
Tao Q, He H, Li T et al (2014) Tailoring surface properties and structure of layered double hydroxides using silanes with different number of functional groups. J solid state Chem 213:176–181
Wang B, Zhang H, Evans DG, Duan X (2005) Surface modification of layered double hydroxides and incorporation of hydrophobic organic compounds. Mater Chem Phys 92:190–196
Zhang Y, Li Y, Ren Y et al (2017) Double-doped LDH films on aluminum alloys for active protection. Mater Lett 192:33–35
Almeda S, Arce L, Valcárcel M (2008) Combination of the solid-phase extraction and large–volume stacking with polarity switching in micellar electrokinetic capillary chromatography for the determination of traces of nonsteriodal anti-inflammatory drugs in saliva. Electrophoresis 29:3074–3080
Roto R (2018) Surface modification of Fe3O4 as magnetic adsorbents for recovery of precious metals. In: Advanced surface engineering research
Food and Drug Administration. US Department of Health and Human Services (2015) Analytical procedures and methods validation for drugs and biologics
Indrayanto G (2018) Validation of chromatographic methods of analysis: application for drugs that derived from herbs. In: profiles drug Subst excipients Relat Methodol 43: 359–392
Moreda-Piñeiro A, Moreda-Piñeiro J, Bermejo-Barrera P (2013) Sample pre–treatment methods for organometallic species determination. In: Speciat stud soil sediment environ samples 19–202
Rahim MA, Ibrahim WAW, Ramli Z, Sanagi MM (2015) New functionalised sol–gel hybrid sorbent coating for stir bar sorptive extraction of selected non–steroidal anti-inflammatory drugs in human urine samples. Malaysian J Anal Sci 19:481–492
Hu C, He M, Chen B, Hu B (2015) Simultaneous determination of polar and apolar compounds in environmental samples by a polyaniline/hydroxyl multi–walled carbon nanotubes composite–coated stir bar sorptive extraction coupled with high performance liquid chromatography. J Chromatogr A 1394:36–45
Fan W, Mao X, He M et al (2014) Development of novel sol–gel coatings by chemically bonded ionic liquids for stir bar sorptive extraction—application for the determination of NSAIDS in real samples. Anal Bioanal Chem 406:7261–7273
Wan Ibrahim WA, Abdul Keyon AS, Prastomo N, Matsuda A (2011) Synthesis and characterization of polydimethylsiloxane–cyanopropyltriethoxysilane–derived hybrid coating for stir bar sorptive extraction. J Sol Gel Sci Technol 59:128–134
Silva ARM, Portugal FCM, Nogueira JMF (2008) Advances in stir bar sorptive extraction for the determination of acidic pharmaceuticals in environmental water matrices. Comparison between polyurethane and polydimethylsiloxane polymeric phases. J Chromatogr A 1209:10–16
Racamonde I, Rodil R, Quintana JB et al (2015) Fabric phase sorptive extraction: a new sorptive microextraction technique for the determination of non–steroidal anti–inflammatory drugs from environmental water samples. Anal Chim Acta 865:22–30
Aparicio I, Martín J, Santos JL et al (2017) Stir bar sorptive extraction and liquid chromatography–tandem mass spectrometry determination of polar and non–polar emerging and priority pollutants in environmental waters. J Chromatogr A 1500:43–52
Hashemi SH, Monfaredzadeh Z (2019) Molecularly imprinted stir bar sorptive extraction coupled with high–performance liquid chromatography for trace analysis of diclofenac in different real samples. Iran J Chem Chem Eng 38:173–183
Acknowledgments
The authors gratefully acknowledge the financial support provided by K.N. Toosi University of Technology (Tehran, Iran).
Author contribution statement
M.T.M.: conceptualization, methodology, validation, formal analysis, writing—original draft, visualization. S.S.: conceptualization, methodology, validation, software, resources, writing—review and editing, visualization, supervision, project administration. Y.R.: methodology, formal analysis, investigation. M.M.: formal analysis, writing—review and editing, validation. M.S.: methodology, investigation, resources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures in this study involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards and performed with permission from the Educational and Research Committee (K. N. Toosi University of Technology) under the direct supervision of the Local Medical Clinic of the university (Approval Number 150796-L/28754).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2358 kb)
Rights and permissions
About this article
Cite this article
Mirzaee, M.T., Seidi, S., Razeghi, Y. et al. In-tube stir bar sorptive extraction based on 3-aminopropyl triethoxysilane surface-modified Ce-doped ZnAl layered double hydroxide thin film for determination of nonsteroidal anti-inflammatory drugs in saliva samples. Microchim Acta 187, 528 (2020). https://doi.org/10.1007/s00604-020-04489-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04489-z