Abstract
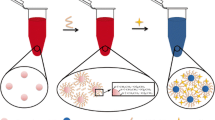
As a first of its kind, we developed a highly sensitive colorimetric nanoprobe for phytic acid (PA) and Fe(III) ion detection based on 4-mercaptophenol (4MP) and thioglycolic acid (TGA)-functionalized gold nanoparticles {AuNPs@(4MP-TGA)}. AuNPs were easily derivatized by 4MP and TGA through –SH binding to gold. Fe(III) ions possibly are bound first to the phenolate groups of 4MP-AuNPs, and further coordinated several nanoparticles via the carboxylate groups of TGA-AuNPs to cause aggregation, resulting in a red-to-purple color change and a bathochromic shift in the SPR absorption band of the nanoprobe. With the addition of PA to the AuNPs@(4MP-TGA)-Fe(III) system, the aggregated particles were released due to strong complex formation between Fe(III) and PA, resulting in a restoration of the color (purple-to-red) and of the SPR band to the original 520 nm wavelength maximum. Thus, the 650-nm absorption is attenuated and the 520-nm band is enhanced upon PA-Fe(III) chelation. This means that the absorption ratio A650/A520 is an indication of Fe(III) whereas the reverse ratio A520/A650 of the PA content of complex samples. The limits of detection (LOD) of the AuNPs@(4MP-TGA) were 1.0 μM for Fe(III) ions and 0.15 μM for PA. Phytic acid extracted from bean grains was determined with the proposed probe, yielding good recoveries. In addition, common metal ions, anions, and several biomolecules did not show an adverse effect on the nanoprobe performance for ferric ions and phytate. The developed method was statistically validated against a LC–MS/MS literature method.

Mercaptophenolate (4MP)- and thioglycolic acid (TGA)-functionalized gold nanoparticles were prepared as nanoprobes to detect Fe(III) ions through nanoparticle aggregation accompanied by red-to-purple color shift. The same nanoprobe determined phytic acid in food through disaggregation of Fe(III)-aggregated nanoparticles by strong Fe(III)-phytate chelation and restoration of solution color from purple to red.









Similar content being viewed by others
References
March JG, Simonet BM, Grases (2001) Determination of phytic acid by gas chromatography-mass spectroscopy: application to biological samples. J Chromatogr B 757:247–255. https://doi.org/10.1016/S0378-4347(01)00155-4
Chen Y, Chen J, Ma K, Cao S, Chen X (2007) Fluorimetric determination of phytic acid in urine based on replacement reaction. Anal Chim Acta 605:185–191. https://doi.org/10.1016/j.aca.2007.10.041
Shi L, Arntfield SD, Nickerson M (2018) Changes in levels of phytic acid, lectins and oxalates during soaking and cooking of Canadian pulses. Food Res Int 107:660–668. https://doi.org/10.1016/j.foodres.2018.02.056
Schlemmer U, Frolich W, Prieto RM, Grases F (2009) Phytate in foods and significance for humans: food sources, intake, processing, bioavailability, protective role and analysis. Mol Nutr Food Res 53:330–375. https://doi.org/10.1002/mnfr.200900099
Zhang W, Gu H, Xi L, Zhang Y, Hu Y, Zhng T (2012) Preparation of phytic acid and its characteristics copper inhibitor. Energy Procedia 17:1641–1647. https://doi.org/10.1016/j.egypro.2012.02.292
Li L, Fu Q, Xia M, Xin L, Shen H, Li G, Xie Y (2018) Inhibition of P-glycoprotein mediated efflux in Caco-2 cells by phytic acid. J Agric Food Chem 66:988–998. https://doi.org/10.1021/acs.jafc.7b04307
Park HR, Ahn HJ, Kim SH, Lee CH, Byun MW, Lee GW (2006) Determination of the phytic acid levels in infant foods using different analytical methods. Food Control 17:727–732. https://doi.org/10.1016/j.foodcont.2005.05.007
Liu T, He L, Valiente M, Lopez-Mesas M (2017) Fast determination of bioactive phytic acid and pyrophosphate in walnuts using microwave accelerated extraction. Food Chem 221:771–775. https://doi.org/10.1016/j.foodchem.2016.11.105
Foster SR, Dilworth LL, Omoruyi FO, Thompson R, Alexander-Lindo RL (2017) Pancreatic and renal function in streptozotocin-induced type 2 diabetic rats administered combined inositol hexakisphosphate and inositol supplement. Biomed Pharmacother 96:72–77. https://doi.org/10.1016/j.biopha.2017.09.126
Macbeth MR, Schubert HL, VanDemark AP, Lingam AT, Hill CP, Bass BL (2005) Inositol hexakisphosphate is bound in the ADAR2 core and required for RNA editing. Science 309:1534–1539. https://doi.org/10.1126/science.1113150
Dost K, Tokul O (2006) Determination of phytic acid in wheat and wheat products by reverse phase high performance liquid chromatography. Anal Chim Acta 558:22–27. https://doi.org/10.1016/j.aca.2005.11.035
Mak WC, Ng YM, Chan C, Kwong WK, Renneberg R (2004) Novel biosensors for quantitative phytic acid and phytase measurement. Biosens Bioelectren 19:1029–1035. https://doi.org/10.1016/j.bios.2003.10.005
Graf E, Mahoney JR, Bryant RG, Eaton JW (1984) Iron-catalyzed hydroxyl radical formation: stringent requirement for free iron coordination site. J Biol Chem 259:3620–3624
Lopez HW, Leenhardt F, Coudray C, Remesy C (2002) Minerals and phytic acid interactions: is it a real problem for human nutrition? Int J Food Sci Technol 37:727–739. https://doi.org/10.1046/j.1365-2621.2002.00618.x
Perello J, Isern B, Munoz JA, Valiente M, Grases F (2004) Determination of phytate in urine by high-performance liquid chromatography-mass spectrometry. Chromatographia 20:265–268. https://doi.org/10.1365/s10337-004-0379-5
Dost K, Karaca G (2016) Evaluation of phytic acid content of some tea and nut products by reserve-phase high performance liquid chromatography/visible detector. Food Anal Methods 9:1391–1397. https://doi.org/10.1007/s12161-015-0319-z
Munoz JA, Valiente M (2003) Determination of phytic acid in urine by inductively coupled plasma mass spectrometry. Anal Chem 75:6374–6378. https://doi.org/10.1021/ac0345805
Rodrigues VC, de Moraes ML, Brisolari A, Soares JC, Ferreira M, Gonçalves D (2003) Polypyrrole/phytase amperometric biosensors for the determination of phytic acid in standard solutions. Sensor Actuators B Chem 160:222–226. https://doi.org/10.1016/j.snb.2011.07.038
Kolozsvari B, Firth S, Saiardi A (2015) Raman spectroscopy detection of phytic acid in plant seeds reveals the absence of inorganic polyphosphate. Mol Plant 8:826–828 https://doi.org/10.1016/j.molp.2015.01.015
Wang Q, Ding C, Zhou Y, Luo Z, Li J (2018) Universal and biocompatible hydroxypatite coating induced by phytic acid-metal complex multilayer. Colloids Surf B Biointerfaces 169:478–485. https://doi.org/10.1016/j.colsurfb.2018.05.057
Cao SH, Dong NA, Chen J (2011) Synchronous fluorescence determination of phytic acid in foodstuffs and urine based on replacement reaction. Phytochem Anal 22:119–123. https://doi.org/10.1002/pca.1254
Ular N, Üzer A, Durmazel S, Erçağ E, Apak R (2018) Diaminocyclohexane-functionalized/thioglycolic acid-modified gold nanoparticle-based colorimetric sensing of trinitrotoluene and tetryl. ACS Sensors 3:2355–2342. https://doi.org/10.1021/acssensors.8b00709
Tur F, Tur E, Lentheric I, Mendoza P, Encabo M, Isern B, Perello J (2013) Validation of an LC-MS bioanalytical method for quantification of phytate levels in rat, dog and human plasma. J Chromatogr B 928:146–154. https://doi.org/10.1016/j.jchromb.2013.03.023
Diouf-Lewis A, Commereuc S, Verney V (2017) Toward greener polyolefins: antioxidant effect of phytic acid from cereal waste. Eur Polym J 96:190–199. https://doi.org/10.1016/j.eurpolymj.2017.09.014
Turkevich J, Stevenson PC, Hillier JA (1951) Study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc 11:55–75. https://doi.org/10.1039/DF9511100055
Chen Y, Chen J, Luo Z, Ma K, Chen X (2009) Synchronous fluorescence analysis of phytate in food. Microchim Acta 164:35–40. https://doi.org/10.1007/s00604-008-0026-1
Wali LA, Hasan KK, Alwan AM (2019) Rapid and highly efficient detection of ultra-low concentration of penicillin G by gold nanoparticles/porous silicon SERS active substrate. Spectrochim Acta A Mol Biomol Spectrosc 206:31–36. https://doi.org/10.1016/j.saa.2018.07.103
Wali LA, Hasan KK, Alwan AM (2020) An investigation of efficient detection of ultra-low concentration of penicillins in milk using AuNPs/PSi hybrid structure. Plasmonics 15:1–9. https://doi.org/10.1007/s11468-019-01096-4
Nguyen KC (2012) Quantitative analysis of COOH-terminated alkanethiol SAMs on gold nanoparticle surfaces. Adv Nat Sci Nanosci Nanotechnol 3:1–5. https://doi.org/10.1088/2043-6262/3/4/045008
Memon SS, Nafady A, Solangi AR, Al-Enizi AM, Sirajuddin SMR, Sherazi STH, Memon S, Arain M, Abrof MI, Khattak MI (2018) Sensitive and selective aggregation based colorimetric sensing of Fe3+ via interaction with acetyl salicylic acid derived gold nanoparticles. Sensor Actuators B Chem 259:1006–1012. https://doi.org/10.1016/j.snb.2017.12.162
Danehy JP, Parameswaran KN (1968) The acidic dissociation constants of a number of thiols have been determined and collated with those already recorded in the literature. J Chem Eng Data 13:386–389. https://doi.org/10.1021/je60038a025
Sakloetsakun D, Hombach JM, Bernkop-Schnürch A (2009) In situ gelling properties of chitosan-thioglycolic acid conjugate in the presence of oxidizing agents. Biomaterials 30:6151–6157. https://doi.org/10.1016/j.biomaterials.2009.07.060
Shears SB (2015) Inositol pyrophosphates: why so many phosphates? Adv Biol Regul 57:203–216. https://doi.org/10.1016/j.jbior.2014.09.015
Evans WJ, McCourtney EJ, Shrager RI (1982) Titration studies of phytic acid. J Am Oil Chem Soc 59:189–191. https://doi.org/10.1007/BF02680274
Crea F, De Stefano C, Milea D, Sammartano S (2008) Formation and stability of phytate complexes in solution. Coord Chem Rev 252:1108–1120. https://doi.org/10.1016/j.ccr.2007.09.008
Torres J, Domínguez S, Cerdá MF, Obal G, Mederos A, Irvine RF, Díaz A, Kremer C (2005) Solution behaviour of myo-inositol hexakisphosphate in the presence of multivalent cations. Prediction of a neutral pentamagnesium species under cytosolic/nuclear conditions. J Inorg Biochem 99:828–840. https://doi.org/10.1016/j.jinorgbio.2004.12.011
Gouger S, Stuehr J (1974) Kinetics of Iron(III) interactions with phenol and o-aminophenol. Inorg Chem 13:379–384
Gao Z, Wang L, Su R, Huang R, Qi W, He Z (2015) A carbon dot-based "off-on" fluorescent probe for highly selective and sensitive detection of phytic acid. Biosens Bioelectron 70:232–238. https://doi.org/10.1016/j.bios.2015.03.043
Graf E, Empson KL, Eaton JW (1987) Phytic acid, a natural antioxidant. J Biol Chem 262:11647–11650
Crea F, Stefano CD, Milea D, Sammartano S (2008) Formation and stability of phytate complexes in solution. Coord Chem 252:1108–1120. https://doi.org/10.1016/j.ccr.2007.09.008
Alwana AM, Yousif AA, Walic LA (2017) The growth of the silver nanoparticles on the mesoporous silicon and macroporous silicon: a comparative study. Indian J Pure Appl Phys 55:813–820
Wali LA, Alwan AM, Dheyab AB, Hashim DA (2019) Excellent fabrication of Pd-Ag NPs/PSi photocatalyst based on bimetallic nanoparticles for improving methylene blue photocatalytic degradation. Optik 179:708–717. https://doi.org/10.1016/j.ijleo.2018.11.011
Atkins RC (1975) Colorimetric determination of Iron in vitamin supplement tablets a general chemistry experiment. J Chem Educ 52:550
Nitin N, LaConte LEW, Zurkiya O, Hu X, Bao G (2004) Functionalization and peptide-based delivery of magnetic nanoparticles as an intracellular MRI contrast agent. J Biol Inorg Chem 9:706–712. https://doi.org/10.1007/s00775-004-0560-1
Osunlaja AA, Idris SO, Iyun JF (2012) Mechanism of the reduction of methylene blue by thiourea in aqueous acidic medium. Int J ChemTech Res 4:609–617
Zhang X, Servos MR, Liu J (2012) Ultrahigh nanoparticle stability against salt, pH, and solvent with retained surface accessibility via depletion stabilization. J Am Chem Soc 134:9910–9913. https://doi.org/10.1021/ja303787e
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 3.10 MB)
Rights and permissions
About this article
Cite this article
Koç, Ö.K., Üzer, A. & Apak, R. A colorimetric probe based on 4-mercaptophenol and thioglycolic acid-functionalized gold nanoparticles for determination of phytic acid and Fe(III) ions. Microchim Acta 187, 586 (2020). https://doi.org/10.1007/s00604-020-04478-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04478-2