Abstract
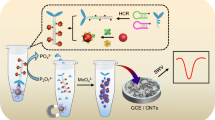
A novel label-free and exonuclease III (Exo III)–assisted signal amplification electrochemical aptasensor was constructed for the determination of carcinoembryonic antigen (CEA) via magnetic field–induced self-assembly of magnetic biocomposites (Fe3O4@Au NPs-S1-S2-S3). The magnetic biocomposites were acquired by modifying double-stranded DNA (S1-S2-S3) on the surface of Fe3O4@Au nanoparticles (Fe3O4@Au NPs). Among them, Fe3O4@Au NPs were used as carriers for magnetic separation, thiolated single-stranded DNA (S1) provided signal sequence, CEA aptamer (S2) worked as a recognition element, and complementary strand (S3) was used to form double strands. In the presence of CEA, S2 bonded with CEA competitively; the exposed S1 could not be cleaved since Exo III was inactive against ssDNA. The G-quadruplex/hemin complexes finally formed with the existence of K+, and the high electrochemical signal of G-quadruplex/hemin complexes was recorded by differential pulse voltammetry (DPV) at − 0.6 V. Conversely, in the absence of CEA, dsDNA was cleaved from the 3′ blunt end by Exo III; the disappearance of G-rich sequence blocked the generation of the signal. This method exhibited good selectivity and sensitivity for the determination of CEA; the linear range was from 0.1 to 200 ng mL−1 and the limit of detection was 0.4 pg mL−1.

Graphical abstract





Similar content being viewed by others
References
Carneiro MCCG, Sousa-Castillo A, Correa-Duarte MA, Sales MGF (2019) Dual biorecognition by combining molecularly-imprinted polymer and antibody in SERS detection. Application to carcinoembryonic antigen. Biosens Bioelectron 146:111761
Wang C, Wang Y, Zhang H, Deng H, Xiong X, Li C, Li W (2019) Molecularly imprinted photoelectrochemical sensor for carcinoembryonic antigen based on polymerized ionic liquid hydrogel and hollow gold nanoballs/MoSe2 nanosheets. Anal Chim Acta 1090:64–71
Tang D, Ren J (2008) In situ amplified electrochemical immunoassay for carcinoembryonic antigen using horseradish peroxidase-encapsulated nanogold hollow microspheres as labels. Anal Chem 80(21):8064–8070
Zhong Z, Wu W, Wang D, Wang D, Shan J, Qing Y, Zhang Z (2010) Nanogold-enwrapped graphene nanocomposites as trace labels for sensitivity enhancement of electrochemical immunosensors in clinical immunoassays: carcinoembryonic antigen as a model. Biosens Bioelectron 25(10):2379–2383
Li J, Cao Y, Hinman SS, McKeating KS, Guan Y, Hu X et al (2018) Efficient label-free chemiluminescent immunosensor based on dual functional cupric oxide nanorods as peroxidase mimics. Biosens Bioelectron 100:304–311
Lin X, Wang Y, Wang L, Lu Y, Li J, Lu D, Zhou T, Huang Z, Huang J, Huang H, Qiu S, Chen R, Lin D, Feng S (2019) Interference-free and high precision biosensor based on surface enhanced Raman spectroscopy integrated with surface molecularly imprinted polymer technology for tumor biomarker detection in human blood. Biosens Bioelectron 143:111599
Shahbazi N, Hosseinkhani S, Ranjbar B (2017) A facile and rapid aptasensor based on split peroxidase DNAzyme for visual detection of carcinoembryonic antigen in saliva. Sensors Actuators B Chem 253:794–803
Zhang Y, Ye W, Yang C, Xu Z (2019) Simultaneous quantitative detection of multiple tumor markers in microfluidic nanoliter-volume droplets. TALANTA 205:120096
Zhu W, Gu C, Dunevall J, Ren L, Zhou X, Ewing AG (2019) Combined amperometry and electrochemical cytometry reveal differential effects of cocaine and methylphenidate on exocytosis and the fraction of chemical release. Angew Chem Int Ed 58(13):4238–4242
Freitas M, Sá Couto M, Barroso MF, Pereira C, De-los-Santos-Álvarez N, Miranda-Ordieres AJ et al (2016) Highly monodisperse Fe3O4@Au superparamagnetic nanoparticles as reproducible platform for genosensing genetically modified organisms. ACS Sensors 1(8):1044–1053
Cabrera FC, Melo AFAA, de Souza JCP, Job AE, Crespilho FN (2015) A flexible lab-on-a-chip for the synthesis and magnetic separation of magnetite decorated with gold nanoparticles. Lab Chip 15(8):1835–1841
Miao P, Tang Y, Wang L (2017) DNA modified Fe3O4@Au magnetic nanoparticles as selective probes for simultaneous detection of heavy metal ions. ACS Appl Mater Interfaces 9(4):3940–3947
Zhang Y, Li H, Chen M, Fang X, Pang P, Wang H, Wu Z, Yang W (2017) Ultrasensitive electrochemical biosensor for silver ion based on magnetic nanoparticles labeling with hybridization chain reaction amplification strategy. Sensors Actuators B Chem 249:431–438
Shi X, Wang Y, Peng C, Zhang Z, Chen J, Zhou X, Jiang H (2017) Enantiorecognition of tyrosine based on a novel magnetic electrochemical chiral sensor. Electrochim Acta 241:386–394
Wang J, Li B, Lu Q, Li X, Weng C, Yan X, et al. A versatile fluorometric aptasensing scheme based on the use of a hybrid material composed of polypyrrole nanoparticles and DNA-silver nanoclusters: application to the determination of adenosine, thrombin, or interferon-gamma. Microchim Acta 2019;186(6)
Sun X, Pan Q, Yuan C, Wang Q, Tang X, Ding K et al (2016) A single ssDNA aptamer binding to mannose-capped lipoarabinomannan of bacillus Calmette–Guérin enhances immunoprotective effect against tuberculosis. J Am Chem Soc 138(36):11680–11689
Liu H, Ma C, Zhou M, Chen H, He H, Wang K (2016) Quencher-free fluorescence strategy for detection of DNA methyltransferase activity based on exonuclease III-assisted signal amplification. Anal Bioanal Chem 408(28):8111–8116
Zheng J, Peng X, Wang Y, Bao T, Wen W, Zhang X, Wang S (2019) An exonuclease-assisted triple-amplified electrochemical aptasensor for mucin 1 detection based on strand displacement reaction and enzyme catalytic strategy. Anal Chim Acta 1086:75–81
Zhou D, Zeng L, Pan J, Li Q, Chen J (2020) Autocatalytic DNA circuit for Hg2+ detection with high sensitivity and selectivity based on exonuclease III and G-quadruplex DNAzyme. TALANTA 207:120258
Li X, Yang J, Yuan R, Xiang Y (2019) Programming cascaded recycling amplifications for highly sensitive and label-free electrochemical sensing of transcription factors in tumor cells. Biosens Bioelectron 142:111574
Kim BG, Long J, Dubins DN, Chalikian TV (2016) Ionic effects on VEGF G-quadruplex stability. J Phys Chem B 120(22):4963–4971
Wu J, Zeng L, Li N, Liu C, Chen J (2019) A wash-free and label-free colorimetric biosensor for naked-eye detection of aflatoxin B1 using G-quadruplex as the signal reporter. Food Chem 298:125034
Lei S, Xu L, Liu Z, Zou L, Li G, Ye B (2019) An enzyme-free and label-free signal-on aptasensor based on DNAzyme-driven DNA walker strategy. Anal Chim Acta 1081:59–64
Zhao W, Liu M, Li H, Wang S, Tang S, Kong R et al (2019) Ultra-sensitive label-free electrochemical detection of the acute leukaemia gene Pax-5a based on enzyme-assisted cycle amplification. Biosens Bioelectron 143:111593
Zhu C, Zhu W, Xu L, Zhou X (2019) A label-free electrochemical aptasensor based on magnetic biocomposites with Pb2+−dependent DNAzyme for the detection of thrombin. Anal Chim Acta 1047:21–27
Han Q, Shen X, Zhu W, Zhu C, Zhou X, Jiang H (2016) Magnetic sensing film based on Fe3O4@Au-GSH molecularly imprinted polymers for the electrochemical detection of estradiol. Biosens Bioelectron 79:180–186
Chen Y, Wu T, Xing G, Kou Y, Li B, Wang X et al (2019) Fundamental formation of three-dimensional Fe3O4 microcrystals and practical application in anchoring Au as recoverable catalyst for effective reduction of 4-nitrophenol. Ind Eng Chem Res 58(33):15151–15161
Guo H, Wang W, Zhou F. Fast and highly selective separation of His-tagged proteins by Ni2+−carrying magnetic core–shell nanoparticles. Applied Physics A 2019;125(5)
Rasouli E, Basirun WJ, Johan MR, Rezayi M, Darroudi M, Shameli K, Shanavaz Z, Akbarzadeh O, Izadiyan Z (2019) Facile and greener hydrothermal honey-based synthesis of Fe3O4/Au core/shell nanoparticles for drug delivery applications. J Cell Biochem 120(4):6624–6631
Lotfi S, Veisi H (2019) Pd nanoparticles decorated poly-methyldopa@GO/Fe3O4 nanocomposite modified glassy carbon electrode as a new electrochemical sensor for simultaneous determination of acetaminophen and phenylephrine. Mater Sci Eng C 105:110112
Wasfi AS, Humud HR, Fadhil NK (2019) Synthesis of core-shell Fe3O4-Au nanoparticles by electrical exploding wire technique combined with laser pulse shooting. Opt Laser Technol 111:720–726
Zhang X, Ding S (2017) Sandwich-structured electrogenerated chemiluminescence immunosensor based on dual-stabilizers-capped CdTe quantum dots as signal probes and Fe3O4-Au nanocomposites as magnetic separable carriers. Sensors Actuators B Chem 240:1123–1133
Deng X, Lin K, Chen X, Guo Q, Yao P (2013) Preparation of magnetic Fe3O4/Au composites for extraction of benzo[a]pyrene from aqueous solution. Chem Eng J 225:656–663
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 81773681, 81572081, 21904069) and the Natural Science Foundation of Jiangsu Province (No. BK20190653). This work was also supported by China Postdoctoral Science Foundation (Nos. 2019M661777, 2017M621789) and the Postdoctoral Science Foundation of Jiangsu (Nos. 2019K068, 2019Z176).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 747 kb)
Rights and permissions
About this article
Cite this article
Li, X., Weng, C., Wang, J. et al. A label-free electrochemical magnetic aptasensor based on exonuclease III–assisted signal amplification for determination of carcinoembryonic antigen. Microchim Acta 187, 492 (2020). https://doi.org/10.1007/s00604-020-04457-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04457-7