Abstract
A stable and enzyme-free method is described for highly sensitive determination of telomerase activity. It is based on the use of a framework nucleic acid (FNA) nanomachine and doxorubicin-spherical nucleic acid (DSNA) tags. Upon incubation with telomerase, the primer-tetrahedron becomes elongated to form the handed swing arm. The extended swing arm autonomously moves along the predefined track consisting of entropy-tetrahedron by consecutive strand displacement under the aid of fuel-tetrahedron. As a result, many (entropy-tetrahedron)-(fuel-tetrahedron) complexes are assembled for combining the DSNA tags. This results in an amplified electrochemical signal, typically measured at around −0.63 V (Ag/AgCl). The use of an enzyme-free FNA nanomachine and of DSNA tags warrants outstandingly high stability and sensitivity. The method shows a broad dynamic correlation of telomerase activity in cell extracts. The analytical range extends from 10 to 1.0 × 104 HeLa cells mL−1 with a lower detection limit of 2 cells mL−1. The differences in telomerase activity between different cancer cells can be easily evaluated. The method was further verified by quantifying telomerase activity of cancer cells in accumulated normal cells. Therefore, the sensing method has great potential for clinical application.

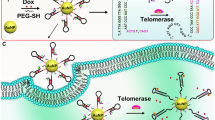
Schematic representation of the electrochemical biosensor based on target induced framework nucleic acid nanomachine with doxorubicin-spherical nucleic acids (DSNA) tags, which can be used to the determination of telomerase activity in accumulated normal cells. dNTP: Deoxynucleotide triphosphates; FT: Fuel-tetrahedron.






Similar content being viewed by others
References
Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR (2007) Protein composition of catalytically active human telomerase from immortal cells. Science 315:1850–1853
Shay JW, Wright WE (2019) Telomeres and telomerase: three decades of progress. Nat Rev Genet 20:299–309
Wang LJ, Ma F, Tang B, Zhang CY (2017) Sensing telomerase: from detection to imaging. Chem Sci 8:2495–2502
Zhou XM, Xing D (2012) Assays for human telomerase activity: progress and prospects. Chem Soc Rev 41:4643–4656
Lee ST, Rahman R, Muthoosamy K, Mohamed NAH, Su X, Tayyab S, New SY (2019) Amplification-free and direct fluorometric determination of telomerase activity in cell lysates using chimeric DNA-templated silver nanoclusters. Microchim Acta 186:81
Ma F, Wang TT, Jiang L, Zhang CY (2019) Ultrasensitive detection of telomerase activity in lung cancer cells with quencher-free molecular beacon-assisted quadratic signal amplification. Anal Chim Acta 1053:112–130
Peng M, Na N, Ouyang J (2019) A fluorescence light-up silver nanocluster beacon modulated by metal ions and its application in telomerase-activity detection. Chemistry 25(14):3598–3605
Yang H, Liu A, Wei M, Liu Y, Lv B, Wei W, Zhang Y, Liu S (2017) Visual, label-free telomerase activity monitor via enzymatic etching of gold nanorods. Anal Chem 89:12094–12100
Wang D, Guo R, Wei Y, Zhang Y, Zhao X, Xu Z (2018) Sensitive multicolor visual detection of telomerase activity based on catalytic hairpin assembly and etching of Au nanorods. Biosens Bioelectron 122:247–253
Meng F, Xu Y, Dong W et al (2018) A PCR-free voltammetric telomerase activity assay using a substrate primer on a gold electrode and DNA-triggered capture of gold nanoparticles. Microchim Acta 185:398
Wang G, Wang H, Cao S, Xiang W, Li T, Yang M (2019) Electrochemical determination of the activity and inhibition of telomerase based on the interaction of DNA with molybdate. Microchim Acta 186:96
Zhang X, Lou X, Xia F (2017) Advances in the detection of telomerase activity using isothermal amplification. Theranostics 7:1847–1862
Kim J, Jang D, Park H, Jung S, Kim DH, Kim WJ (2018) Functional-DNA-driven dynamic nanoconstructs for biomolecule capture and drug delivery. Adv Mater 30:1707351
Scalise D, Schulman R (2019) Controlling matter at the molecular scale with DNA circuits. Annu Rev Biomed Eng 21:469–493
Wang H, Wang H, Liu C, Duan X, Li Z (2016) Ultrasensitive detection of telomerase activity in a single cell using stem-loop primer-mediated exponential amplification (SPEA) with near zero nonspecific signal. Chem Sci 7:4945–4950
Li CC, Zhang Y, Liu WJ, Zhang CY (2018) A triple-amplification strategy for sensitive detection of telomerase at the single-cell level. Chem Commun 54:9317–9320
Wang W, Huang S, Li J, Rui K, Bi S, Zhang JR, Zhu JJ (2017) Evaluation of intracellular telomerase activity through cascade DNA logic gates. Chem Sci 8:174–180
Xu X, Wang L, Li K, Huang Q, Jiang W (2018) A smart DNA tweezer for detection of human telomerase activity. Anal Chem 90:3521–3530
Wang Z, Hou R, Loh IY (2019) Track-walking molecular motors: a new generation beyond bridge-burning designs. Nanoscale 11:9240–9263
Huang J, Zhu L, Ju H, Lei J (2019) Telomerase triggered DNA Walker with a superhairpin structure for human telomerase activity sensing. Anal Chem 91:6981–6985
Li X, Cheng W, Li D, Wu J, Ding X, Cheng Q, Ding S (2016) A novel surface plasmon resonance biosensor for enzyme-free and highly sensitive detection of microRNA based on multi component nucleic acid enzyme (MNAzyme)-mediated catalyzed hairpin assembly. Biosens Bioelectron 80:98–104
Yang F, Li Q, Wang L, Zhang GJ, Fan C (2018) Framework-nucleic-acid-enabled biosensor development. ACS Sens 3:903–919
Ge Z, Gu H, Li Q, Fan C (2018) Concept and development of framework nucleic acids. J Am Chem Soc 140:17808–17819
Ye D, Zuo X, Fan C (2018) DNA nanotechnology-enabled interfacial engineering for biosensor development. Annu Rev Anal Chem 11:171–195
Bui H, Shah S, Mokhtar R, Song T, Garg S, Reif J (2018) Localized DNA hybridization chain reactions on DNA origami. ACS Nano 12:1146–1155
He L, Lu D, Liang H, Xie S, Zhang X, Liu Q, Yuan Q, Tan W (2018) mRNA-initiated, three-dimensional DNA amplifier able to function inside living cells. J Am Chem Soc 140:258–263
Yan T, Zhu L, Ju H, Lei J (2018) DNA-Walker-induced allosteric switch for tandem signal amplification with palladium nanoparticles/metal-organic framework tags in electrochemical biosensing. Anal Chem 90:14493–14499
Chinen AB, Guan CM, Ko CH, Mirkin CA (2017) The impact of protein corona formation on the macrophage cellular uptake and biodistribution of spherical nucleic acids. Small 13(16):1603847
Zhang K, Hao L, Hurst SJ, Mirkin CA (2012) Antibody-linked spherical nucleic acids for cellular targeting. J Am Chem Soc 134(40):16488–16491
Tan X, Lu X, Jia F, Liu X, Sun Y, Logan JK, Zhang K (2016) Blurring the role of oligonucleotides: spherical nucleic acids as a drug delivery vehicle. J Am Chem Soc 138:10834–10837
Bagalkot V, Farokhzad OC, Langer R, Jon S (2006) An aptamer-doxorubicin physical conjugate as a novel targeted drug-delivery platform. Angew Chem Int Ed Engl 45(48):8149–8152
Liu J, Lu Y (2006) Preparation of aptamer-linked gold nanoparticle purple aggregates for colorimetric sensing of analytes. Nat Protoc 1:246–252
Jiang Q, Song C, Nangreave J, Liu X, Lin L, Qiu D, Wang ZG, Zou G, Liang X, Yan H, Ding B (2012) DNA origami as a carrier for circumvention of drug resistance. J Am Chem Soc 134(32):13396–13403
Qian R, Ding L, Yan L, Lin M, Ju H (2014) Smart vesicle kit for in situ monitoring of intracellular telomerase activity using a telomerase-responsive probe. Anal Chem 86(17):8642–8648
Haiss W, Thanh NT, Aveyard J, Fernig DG (2007) Determination of size and concentration of gold nanoparticles from UV-vis spectra. Anal Chem 79(11):4215–4221
Wang L, Meng T, Yu G, Wu S, Sun J, Jia H, Wang H, Yang X, Zhang Y (2019) A label-free electrochemical biosensor for ultrasensitively detecting telomerase activity based on the enhanced catalytic currents of acetaminophen catalyzed by au nanorods. Biosens Bioelectron 124-125:53–58
Li X, Cui Y, Du Y, Tang A, Kong D (2019) Label-free telomerase detection in single cell using a five-base telomerase product-triggered exponential rolling circle amplification strategy. ACS Sens 4(4):1090–1096
Xiong C, Liang W, Zheng Y, Zhuo Y, Chai Y, Yuan R (2017) Ultrasensitive assay for telomerase activity via Selfenhanced Electrochemiluminescence ruthenium complex doped metal-organic frameworks with high emission efficiency. Anal Chem 89:3222–3227
Kazemi E, Bagheri H, Norouzian D (2019) A turn-on graphene quantum dot and graphene oxide based fluorometric aptasensor for the determination of telomerase activity. Microchim Acta 186(12):785
He C, Liu Z, Wu Q, Zhao J, Liu R, Liu B, Zhao T (2018) Ratiometric fluorescent biosensor for visual discrimination of cancer cells with different telomerase expression levels. ACS Sens 3:757–762
Acknowledgments
This work was supported by Health Commission of Henan Province (No.2018020495).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 321 kb)
Rights and permissions
About this article
Cite this article
Shen, Y., Gong, J., Xu, Q. et al. Target induced framework nucleic acid nanomachine with doxorubicin-spherical nucleic acid tags for electrochemical determination of human telomerase activity. Microchim Acta 187, 97 (2020). https://doi.org/10.1007/s00604-019-4095-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-4095-0