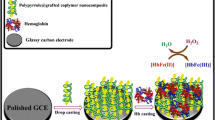
Abstract
A facile and highly sensitive biosensor was developed for the determination of hydrogen peroxide (H2O2) via electrochemical catalytic reduction of H2O2 by hemoglobin (Hb). Hb was enriched and immobilized simply in a chitosan (Chit) membrane on a magnetic electrode to construct an enzyme-like biosensor. The biosensor catalyzes the electrochemical reduction of H2O2 under an external magnetic field. The response improved roughly twice as Hb was adsorbed by Chit in an alkaline medium. The response of the biosensor under the magnetic field increased by 16% owing to the paramagnetism of Hb. The effect of pH values on Hb adsorption by Chit, as well as the effect of an external magnetic field on Hb configuration were investigated by UV-vis spectroscopy. The reduction peak current has linear and log-linear relationships with H2O2 concentration in the range of 5–250 μmol∙L−1 and 0.01–1 μmol∙L−1, respectively. The detection limit was 0.003 μmol∙L−1, with a good sensitivity of 0.227 μA∙μM−1∙cm−2. The biosensor was successfully applied to the determination of H2O2 in milk samples and in disinfectant solutions. Recoveries ranged from 96.3 to 105.4%, and from 95.3 to 107.7%, respectively.

Construction of the biosensor, and principle of H2O2 determination based on Hb bioelectrocatalysis.






Similar content being viewed by others
References
Chen W, Cai S, Ren QQ, Wen W, Zhao YD (2012) Recent advances in electrochemical sensing for hydrogen peroxide: a review. Analyst 13:749–758
Miller EW, Chang CJ (2007) Fluorescent probes for nitric oxide and hydrogen peroxide in cell signaling. Curr Opin Chem Biol 11:620–625
Cinti S, Basso M, Moscone D, Arduini F (2017) A paper-based nanomodified electrochemical biosensor for ethanol detection in beers. Anal Chim Acta 960:123–130
AbellánLlobregat A, Jeerapan I, Bandodkar A, Vidal L, Canals A, Wang J, Morallóna E (2017) A stretchable and screen-printed electrochemical sensor for glucose determination in human perspiration. Biosens Bioelectron 91:885–891
Yamazaki T, Ikeda T, Lim B, Okumura K, Ishida M, Sawada K (2011) Smart Integrated Sensor for Multiple Detections of Glucose and L-Lactate Using On-Chip Electrochemical System. J. Sensors 2011: Article ID 190284
Liu H, Weng L, Yang C (2017) A review on nanomaterial-based electrochemical sensors for H2O2, H2S and NO inside cells or released by cells. Microchim Acta 184:1267–1283
Raja S, Ramesh V, Thivaharan V (2017) Green biosynthesis of silver nanoparticles using Calliandra haematocephala, leaf extract, their antibacterial activity and hydrogen peroxide sensing capability. Arab J Chem 10:253–261
Motaghed RM, Ge L, Jiang H, Wang X (2018) A facile photoelectrochemical sensor for high sensitive ROS and AA detection based on graphitic carbon nitride nanosheets. Biosens Bioelectron 107:54–61
Zangheri M, Cevenini L, Anfossi L, Baggiani C, Simoni P, Nardo FD (2015) A simple and compact smartphone accessory for quantitative chemiluminescence-based lateral flow immunoassay for salivary cortisol detection. Biosens Bioelectron 64:63–68
Zhang LS, Wong GTF (1999) Optimal conditions and sample storage for the determination of H2O2 in marine waters by the scopoletin-horseradish peroxidase fluorometric method. Talanta 48:1031–1038
Lorestani F, Shahnavaz Z, Mn P, Alias Y, Manan NSA (2015) One-step hydrothermal green synthesis of silver nanoparticle-carbon nanotube reduced-graphene oxide composite and its application. Sensor Actuat B-Chem 208:389–398
Amin VM, Olson NF (1967) Spectrophotometric determination of hydrogen peroxide in Milk. J Dairy Sci 50:461–464
Gilliland SE (1969) Enzymatic determination of residual hydrogen peroxide in Milk. J Dairy Sci 52:321–324
Tantawi O, Baalbaki A, Asmar REl, Ghauch A (2019) A rapid and economical method for the quantification of hydrogen peroxide (H2O2) using a modified HPLC apparatus. Sci Total Environ 654: 107–117
Lu J, Kang Q, Xiao JH, Wang T, Fang M, Yu L (2018) Luminescent, stabilized and environmentally friendly [EuW10O36]9−-chitosan films for sensitive detection of hydrogen peroxide. Carbohydr Polym 200:560–566
Sheng WQ, Xu Q, Chen JN, Wang HC, Ying ZH, Gao RF, Zheng XM, Zhao XQ (2019) Electrochemical sensing of hydrogen peroxide using nitrogen-doped Graphene/porous Iron oxide Nanorod composite. Mater Lett 235:137–140
Chen A, Chatterjee S (2013) Nanomaterials based electrochemical sensors for biomedical applications. Chem Soc Rev 42:5425–5438
Shao Y, Wang J, Wu H, Liu J, Aksay I, Lin Y (2010) Graphene based electrochemical sensors and biosensors: a review. Electroanal. 22:1027–1036
Wang J (2010) Carbon-nanotube based electrochemical biosensors: a review. Electroanal. 17:7–14
Bartlett PN, Tebbutt P, Tyrrell CH (2002) Electrochemical immobilization of enzymes. 3. Immobilization of glucose oxidase in thin films of electrochemically polymerized phenols. Anal Chem 64:138–142
Amreen K, Kumar AS (2018) A human whole blood chemically modified electrode for the hydrogen peroxide reduction and sensing: real-time interaction studies of hemoglobin in the red blood cell with hydrogen peroxide. J Electroanal Chem 815:189–197
Feng JJ, Zhao G, Xu JJ, Chen HY (2005) Direct electrochemistry and electrocatalysis of heme proteins immobilized on gold nanoparticles stabilized by chitosan. Anal Biochem 342:280–286
Zhu S, Guo J, Dong J, Cui Z, Lu T, Zhu C, Zhang D, Ma J (2013) Sonochemical fabrication of Fe3O4 nanoparticles on reduced graphene oxide for biosensors. Ultrason Sonochem 20:872–880
Yuan Y, Wang JX, Cao YH (2018) Electrochemical sensor based on magnetic electrode modified with magnetic molecularly imprinted nanoparticles immobilized hemoglobin for determination of hydrogen peroxide. Electrochem. https://doi.org/10.13208/j.electrochem.180704 (in Chinese)
Jasmina A, Valéria G, Olga V, Dániel M, Andrea R, Zoltán K, Kurt K (2016) Hydrodynamic chronoamperometric determination of hydrogen peroxide using carbon paste electrodes coated by multiwalled carbon nanotubes decorated with MnO2, or Pt particles. Sensor Actuat B-Chem 233:83–92
Zolghadri S, Saboury AA, Amin E, Moosavi-Movahedi AA (2010) A spectroscopic study on the interaction between ferric oxide nanoparticles and human hemoglobin. J Iran Chem Soc 7:S145–S153
Sun W, Zhai Z, Li X, Qu L, Zhan T, Jiao K (2009) Direct electrochemistry of hemoglobin in chitosan/multiwalled carbon nanotubes/ionic liquid-modified carbon-paste electrode. Anal Lett 42:2460–2473
Zhao HY, Zheng W, Meng ZX, Zhou HM, Xu XX, Li Z, Zheng YF (2009) Bioelectrochemistry of hemoglobin immobilized on a sodium alginate-multiwall carbon nanotubes composite film. Biosens Bioelectron 24:2352–2357
Yuan Y, Ni XJ, Cao YH (2019) Electrochemical determination of hemoglobin on a magnetic electrode modified with chitosan based on electrocatalysis of oxygen. J Electroanal Chem 837:219–225
Gautam V, Singh KP, Yadav VL (2018) Polyaniline/multiwall carbon nanotubes/starch nanocomposite material and hemoglobin modified carbon paste electrode for hydrogen peroxide and glucose biosensing. Int J Biol Macromol 111:1124–1132
Jian F, Qiao Y, Zhuang R (2007) Direct electrochemistry of hemoglobin in TATP film: application in biological sensor. Sensor Actuat B-Chem 124:413–420
Feng JJ, Xu JJ, Chen HY (2005) Synergistic effect of zirconium phosphate and au nanoparticles on direct electron transfer of hemoglobin on glassy carbon electrode. J Electroanal Chem 585:44–50
Guo F, Xu XX, Sun ZZ, Zhang JX, Meng ZX, Zheng W, Zhou HM, Wang BL, Zheng YF (2011) A novel amperometric hydrogen peroxide biosensor based on electrospun Hb-collagen composite. Colloids Surf B 86:140–145
Wang Y, Tang M, Lin X, Gao F, Li M (2012) Sensor for hydrogen peroxide using a hemoglobin-modified glassy carbon electrode prepared by enhanced loading of silver nanoparticle onto carbon nanospheres via spontaneous polymerization of dopamine. Microchim Acta 176:405–410
Zhang LL, Han GQ, Liu Y, Tang J, Tang WH (2104) Immobilizing haemoglobin on gold/graphene-chitosan nanocomposite as efficient hydrogen peroxide biosensor. Sensors Actuators B Chem 197: 164–171
Schnorr JM, Swager TM (2011) Emerging applications of carbon nanotubes. Chem Mater 23:646–657
Mehdi B, Mona R, Moslem ML (2017) Mohaddeseh Amiri-Aref. Bioelectrocatalysis of hydrogen peroxide based on immobilized hemoglobin onto glassy carbon electrode modified with magnetic poly (indole-co-thiophene) nanocomposite. J Electroanal Chem 784:69–76
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1265 kb)
Rights and permissions
About this article
Cite this article
Yuan, Y., Ni, X. & Cao, Y. A magnetic electrode modified with hemoglobin for determination of hydrogen peroxide: distinctly improved response by applying a magnetic field. Microchim Acta 187, 92 (2020). https://doi.org/10.1007/s00604-019-4061-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-4061-x