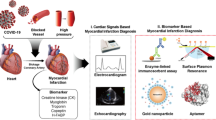
Abstract
An ultra-sensitive method is described here for the determination of HIF-1α (an early biomarker for myocardial infarction) in circulating exosomes in serum. Gold nanospheres were functionalized with a HIF-1α-binding aptamer via sulfydryl chemistry. The apt-AuNP-coated gold seeds were grown by seed-mediated growth, and this significantly increased the peroxidase-mimicking property the nanoparticles. A chromogenic system composed of 3,3′5,5′-tetramethylbenzidine and hydrogen peroxide was used. Absorbance at 652 nm increases linearly in the 0.3 to 200 ng L−1 HIF-1α concentration range, and the limit of detection is 0.2 ng L−1. The method was tested by analyzing rat serum from isoproterenol (ISO)-induced myocardial infarction. It allows HIF-1α to be directly determined in a 25 μL sample without preconcentration. The assay is not interfered by the polydispersity of exosomes released under either health and disease conditions.

Gold nanospheres were functionalized with a HIF-1α-binding aptamer via sulfydryl chemistry. Nanosized gold seed particles were then modified with the functionalized gold nanospheres, and this strongly increases the peroxidase-mimicking activity of the nanomaterial. By using the tetramethylbenzidine/H2O2 chromogenic system, the absorbance at 652 nm increases linearly in the 0.3 to 200 ng L-1 HIF-1α concentration range.





Similar content being viewed by others
Abbreviations
- CVDs:
-
Cardiovascular diseases
- MI:
-
Myocardial infarction
- cTn:
-
Cardiac troponin
- LDH:
-
Lactate dehydrogenase
- CK-MB:
-
Creatine kinase MB
- HIF-1α:
-
Hypoxia-inducible factor-1 alpha
- HRP:
-
Horseradish peroxidase
- ISEV:
-
International Society for Extracellular Vesicles
- AuNPs:
-
Gold nanoparticles
- LOD:
-
Limit of detection
- NTA:
-
Nanoparticle tracking analysis
- DLS:
-
Dynamic light scattering
- TEM:
-
Transmission electron microscope
- CV:
-
Coefficient of variation
- ISO:
-
Isoproterenol hydrochloride
References
Zaman S, Kovoor P (2014) Sudden cardiac death early after myocardial infarction pathogenesis, risk stratification, and primary prevention. Circulation 129:2426–2435
Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, Biedert S, Schaub N, Buerge C, Potocki M, Noveanu M, Breidthardt T, Twerenbold R, Winkler K, Bingisser MC (2009) Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. New Engl J Med 361:858–867
Lang RM, Badano L, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf F, Foster E, Goldstein S, Kuznetsova T, Muraru D, Picard M, Rietzschel E, Rudski L, Spencer K, Tsang W, Voigt J (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J-Card Img 16:233–271
Ghotbi A, Clemmensen A, Kyhl K, Follin B, Hasbak P, Engstrom T, Ripa R, Kjaer A (2019) Rubidium-82 PET imaging is feasible in a rat myocardial infarction model. J Nucl Cardiol 26:798–809
Vita T, Murphy D, Osborne M, Bajaj N, Keraliya A, Jacob S, Martinez A, Nodoushani A, Bravo P, Hainer J, Bibbo C, Steigner M, Taqueti V, Skali H, Blankstein R, Di Carli M, Dorbala S (2019) Association between nonalcoholic fatty liver disease at ct and coronary microvascular dysfunction at myocardial perfusion PET/CT. Radiology 291:329–336
Duan P, Tan J, Miao Y, Zhang Q (2019) Potential role of exosomes in the pathophysiology, diagnosis, and treatment of hypoxic diseases. Am J Transl Res 11:1184–1201
Shah R, Patel T, Freedman J (2018) Circulating extracellular vesicles in human disease. New Engl J Med 379:958–966
Semenza G (2014) Hypoxia-inducible factor 1 and cardiovascular disease. Annu Rev Physiol 76:39–56
Heikal L, Ghezzi P, Mengozzi M, Ferns G (2018) Assessment of HIF-1 alpha expression and release following endothelial injury in-vitro and in-vivo. Mol Med 24:22
Chen H, Sun Y, Li Y, Zhao J, Cao Y (2018) Determination of hypoxia-inducible factor-1 by using a ratiometric colorimetric test based on click-mediated growth of gold nanoparticles. Microchim Acta 185:451
Xuan W, Peng Y, Deng Z, Peng T, Kuai H, Li Y, He J, Jin C, Liu Y, Wang R, Tan W (2018) A basic insight into aptamer-drug conjugates (ApDCs). Biomaterials 182:216–226
Lee J, So H, Jeon E, Chang H, Won K, Kim Y (2008) Aptamers as molecular recognition elements for electrical nanobiosensors. Anal Bioanal Chem 390:1023–1032
Yang R, Mu W, Chen Q, Wang Q, Gao J (2018) Smart magnetic nanoaptamer: construction, subcellular distribution, and silencing HIF for cancer gene therapy. Acs Biomater Sci Eng 4:2606–2613
Zhu H, Zhang L, Liu Y, Zhou Y, Wang K, Xie X, Song L, Wang D, Han C, Chen Q (2016) Aptamer-PEG-modified Fe3O4@Mn as a novel T1-and T2-dual-model MRI contrast agent targeting hypoxia-induced cancer stem cells. Sci Rep 6:12
Mu W, Xiao X, Chen T, Chen Q (2016) Mn(II) silver-aptamer clusters for targeted MR imaging of tumors. J Mater Chem B 4:5284–5288
Zhao T, Chen Q, Wang P, Chen Z (2014) A DNA-Ag cluster as a sensor for BODIPY isomers and HepG-2 cells. RSC Adv 4:10390–10394
Zhao T, Chen Q, Zeng C, Lan Y, Cai J, Liu J, Gao J (2013) Multi-DNA-Ag nanoclusters: reassembly mechanism and sensing the change of HIF in cells. J Mater Chem B 1:4678–4683
Wu J, Wang X, Wang Q, Lou Z, Li S, Zhu Y, Qin L, Wei H (2019) Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem Soc Rev 48:1004–1076
Inkpen M, Liu Z, Li H, Campos L, Neaton J, Venkataraman L (2019) Non-chemisorbed gold-sulfur binding prevails in self-assembled monolayers. Nat Chem 11:351–358
Jadhav S (2012) Functional self-assembled monolayers (SAMs) of organic compounds on gold nanoparticles. J Mater Chem 22:5894–5899
Zhao F, Zhou J, Su X, Wang Y, Yan X, Jia S, Du B (2017) A smart responsive dual aptamers-targeted bubble-generating nanosystem for cancer triplex therapy and ultrasound imaging. Small 13:1603990
Farka Z, Cunderlova V, Horackova V, Pastucha M, Mikusova Z, Hlavacek A, Skladal P (2018) Prussian blue nanoparticles as a catalytic label in a Sandwich Nanozyme-linked Immunosorbent assay. Anal Chem 90:2348–2354
Cunderlova V, Hlavacek A, Hornakova V, Peterek M, Nemecek D, Hampl A, Eyer L (2016) Skladal P (2016) catalytic nanocrystalline coordination polymers as an efficient peroxidase mimic for labeling and optical immunoassays. Microchim Acta 183:651–658
Jiang B, Duan D, Gao L, Zhou M, Fan K, Tang Y, Xi J, Bi Y, Tong Z, Gao GF, Xie N, Tango A, Nie G, Liang M, Yan X (2018) Standardized assays for determining the catalytic activity and kinetics of peroxidase-like nanozymes. Nat Protoc 13:1506–1520
Chen Z, Lai G, Liu S, Yu A (2018) Ultrasensitive electrochemical aptasensing of kanamycin antibiotic by enzymatic signal amplification with a horseradish peroxidase-functionalized gold nanoprobe. Sensor Actuat B-Chem 273:1762–1767
Grabar K, Freeman R, Hommer M, Natan M (1995) Preparation and characterization of au colloid monolayers. Anal Chem 67:735–743
Haiss W, Thanh N, Aveyard J, Fernig D (2007) Determination of size and concentration of gold nanoparticles from UV-Vis spectra. Anal Chem 79:4215–4221
Pavlov V, Xiao Y, Shlyahovsky B, Willner I (2004) Aptamer-functionalized au nanoparticles for the amplified optical detection of thrombin. J Am Chem Soc 126:11768–11769
Théry C, Amigorena S, Raposo G, Clayton A (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 3:22–30
Chen ZH, Chen CQ, Huang HW, Luo F, Guo LH, Zhang L, Lin ZY, Chen GN (2018) Target-induced horseradish peroxidase deactivation for multicolor colorimetric assay of hydrogen sulfide in rat brain microdialysis. Anal Chem 90:6222–6228
van der Pol E, Coumans F, Sturk A, Nieuwland R, Van Leeuwen T (2014) Refractive index determination of nanoparticles in suspension using nanoparticle tracking analysis. Nano Lett 14:6195–6201
Jain P, Mahajan U, Shinde S, Surana S (2018) Cardioprotective role of FA against isoproterenol induced cardiac toxicity. Mol Biol Rep 45:1357–1365
Boulanger C, Loyer X, Rautou P, Amabile N (2017) Extracellular vesicles in coronary artery disease. Nat Rev Cardiol 14:259–272
Acknowledgements
CAS “Light of West China” Program (No. 2018XBZG_XBQNXZ_A_005), Biological Resources Program, Chinese Academy of Sciences (KFJ-BRP-008)
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 403 kb)
Rights and permissions
About this article
Cite this article
Wang, QL., Huang, WX., Zhang, PJ. et al. Colorimetric determination of the early biomarker hypoxia-inducible factor-1 alpha (HIF-1α) in circulating exosomes by using a gold seed-coated with aptamer-functionalized Au@Au core-shell peroxidase mimic. Microchim Acta 187, 61 (2020). https://doi.org/10.1007/s00604-019-4035-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-4035-z