Abstract
Pyrophosphate-modified carbon quantum dots (PP-CDs) are demonstrated to be a viable fluorescent nanoprobe for mercury(II) (Hg2+) detection. Hg2+ reacts with the pyrophosphate groups on the surface of PP-CDs to form a non-fluorescent complex. This results in quenching of the green fluorescence which has excitation/emission peaks at 400/513 nm. Static quenching is shown to be the dominant mechanism. The probe works in 0.1 μM to 1.4 μM Hg2+ concentration range, and the limit of detection is 2 nM. The PP-CDs were also used to visualize Hg2+ inside human hepatocyte LO2 cells.

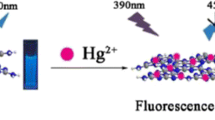
Schematic representation of pyrophosphate-modified carbon quantum dots (CDs) for selective and sensitive fluorometric determination of mercury(II). Hg(II) quenches the blue fluorescence of the CDs, and glutathione restores it. The method was used to detect Hg(II) in spiked tap water and inside cells.






Similar content being viewed by others
References
Joseph J, Anappara AA (2017) Ellagic acid-functionalized fluorescent carbon dots for ultrasensitive and selective detection of mercuric ions via quenching. J Lumin 192:761–766
Altunay N, Gürkan R, Orhan U (2017) Indirect determination of the flavor enhancer maltol in foods and beverages through flame atomic absorption spectrometry after ultrasound assisted-cloud point extraction. Food Chem 235:308–317
Huang RJ, Zhuang ZX, Tai Y, Huang RF, Wang XR, Lee FS (2006) Direct analysis of mercury in traditional Chinese medicines using thermolysis coupled with on-line atomic absorption spectrometry. Talanta 68(3):728–734
Kutscher DJ, Fricker MB, Hattendorf B, Bettmer J, Günther D (2011) Systematic studies on the determination of Hg-labelled proteins using laser ablation-ICPMS and isotope dilution analysis. Anal Bioanal Chem 401(9):2691
Zhou L, Xiong W, Liu S (2015) Preparation of a gold electrode modified with Au–TiO2 nanoparticles as an electrochemical sensor for the detection of mercury (II) ions. J Mater Sci 50(2):769–776
Jia X, Gong D, Han Y, Wei C, Duan TC, Chen HT (2012) Fast speciation of mercury in seawater by short-column high-performance liquid chromatography hyphenated to inductively coupled plasma spectrometry after on-line cation exchange column preconcentration. Talanta 88:724–729
Lim SY, Shen W, Gao Z (2015) Carbon quantum dots and their applications. Chem Soc Rev 44(1):362–381
Berlina AN, Zherdev AV, Dzantiev BB (2019) Progress in rapid optical assays for heavy metal ions based on the use of nanoparticles and receptor molecules. Microchim Acta 186(3):172
Li H, Kang Z, Liu Y, Lee ST (2012) Carbon nanodots: synthesis, properties and applications. J Mater Chem 22(46):24230–24253
Luo PG, Yang F, Yang ST, Sonkar SK, Yang L, Broglie JJ, Sun YP (2014) Carbon-based quantum dots for fluorescence imaging of cells and tissues. RSC Adv 4(21):10791–10807
Xu Q, Li B, Ye Y, Cai W, Li W, Yang C, Street J (2018) Synthesis, mechanical investigation, and application of nitrogen and phosphorus co-doped carbon dots with a high photoluminescent quantum yield. Nano Res 11(7):3691–3701
Li Y, Zhao Y, Cheng H, Hu Y, Shi G, Dai L, Qu L (2011) Nitrogen-doped graphene quantum dots with oxygen-rich functional groups. J Am Chem Soc 134(1):15–18
Luo L, Wang P, Wang Y, Wang F (2018) pH assisted selective detection of Hg (II) and Ag (I) based on nitrogen-rich carbon dots. Sensors Actuators B Chem 273:1640–1647
Ren G, Zhang Q, Li S, Fu S, Chai F, Wang C, Qu F (2017) One pot synthesis of highly fluorescent N doped C-dots and used as fluorescent probe detection for Hg2+ and Ag+ in aqueous solution. Sensors Actuators B Chem 243:244–253
Wang Y, Chang Q, Hu S (2017) Carbon dots with concentration-tunable multicolored photoluminescence for simultaneous detection of Fe3+ and Cu2+ ions. Sensors Actuators B Chem 253:928–933
Tabaraki R, Abdi O (2018) Green and simple turn off/on fluorescence sensor for mercury (II), cysteine and histidine. J Mol Liq 251:77–82
Anh NTN, Chowdhury AD, Doong R (2017) Highly sensitive and selective detection of mercury ions using N, S-codoped graphene quantum dots and its paper strip based sensing application in wastewater. Sensors Actuators B Chem 252:1169–1178
Iqbal A, Iqbal K, Xu L, Li B, Gong D, Liu X, Guo H (2018) Heterogeneous synthesis of nitrogen-doped carbon dots prepared via anhydrous citric acid and melamine for selective and sensitive turn on-off-on detection of Hg (II), glutathione and its cellular imaging. Sensors Actuators B Chem 255:1130–1138
Liao S, Zhu F, Zhao X, Yang H, Chen X (2018) A reusable P, N-doped carbon quantum dot fluorescent sensor for cobalt ion. Sensors Actuators B Chem 260:156–164
Gong X, Lu W, Liu Y, Li Z, Shuang S, Dong C, Choi MM (2015) Low temperature synthesis of phosphorous and nitrogen co-doped yellow fluorescent carbon dots for sensing and bioimaging. J Mater Chem B 3(33):6813–6819
Guo Y, Cao F, Li Y (2018) Solid phase synthesis of nitrogen and phosphor co-doped carbon quantum dots for sensing Fe3+ and the enhanced photocatalytic degradation of dyes. Sensors Actuators B Chem 255:1105–1111
Wang W, Peng J, Li F, Su B, Chen X (2019) Phosphorus and chlorine co-doped carbon dots with strong photoluminescence as a fluorescent probe for ferric ions. Microchim Acta 186(1):32
Chandra S, Chowdhuri AR, Laha D, Laha D, Sahu SK (2018) Fabrication of nitrogen-and phosphorous-doped carbon dots by the pyrolysis method for iodide and iron (III) sensing. Luminescence 33(2):336–344
Yang Y, Huo D, Wu H, Wang X, Yang J, Bian M, Hou C (2018) N, P-doped carbon quantum dots as a fluorescent sensing platform for carbendazim detection based on fluorescence resonance energy transfer. Sensors Actuators B Chem 274:296–303
Ju YJ, Li N, Liu SG, Fan YZ, Ling Y, Xiao N, Li NB (2018) Green synthesis of blue fluorescent P-doped carbon dots for selective determination of picric acid in aqueous medium. Anal Sci 18:372
Grigg ARC, Kretzschmar R, Gilli RS, Wiederhold JG (2018) Mercury isotope signatures of digests and sequential extracts from industrially contaminated soils and sediments. Sci Total Environ 636:1344–1354
Silva PRM, El Khakani MA, Chaker M, Dufresne A, Courchesne F (2011) Simultaneous determination of cd, Pb, and cu metal trace concentrations in water certified samples and soil extracts by means of hg-electroplated-Ir microelectrode array based sensors. Sensors Actuators B Chem 76(1–3):250–257
Hai X, Wang Y, Hao X, Chen X, Wang J (2018) Folic acid encapsulated graphene quantum dots for ratiometric pH sensing and specific multicolor imaging in living cells. Sensors Actuators B Chem 268:61–69
Li LS, Jiao XY, Zhang Y, Cheng C, Huang K, Xu L (2018) Green synthesis of fluorescent carbon dots from Hongcaitai for selective detection of hypochlorite and mercuric ions and cell imaging. Sensors Actuators B Chem 263:426–435
Luo D, Liu SG, Li NB (2018) Water-soluble polymer dots formed from polyethylenimine and glutathione as a fluorescent probe for mercury (II). Microchim Acta 185(6):284
Liu Y, Tang X, Deng M, Cao Y, Li Y, Zheng H, Gao L (2019) Nitrogen doped graphene quantum dots as a fluorescent probe for mercury(II) ions. Microchim Acta 186(3):140
Tan H, Zhang Y, Chen Y (2011) Detection of mercury ions (Hg2+) in urine using a terbium chelate fluorescent probe. Sensors Actuators B Chem 156(1):120–125
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 51505324, 51622507, 81602506), Basic Research Program of Shanxi for Youths (201701D221111), Scientific and Technologial Innovation Programs of Higher Education Institutions in Shanxi (201802036).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 3.16 MB)
Rights and permissions
About this article
Cite this article
Duan, Q., Wang, X., Zhang, B. et al. A fluorometric method for mercury(II) detection based on the use of pyrophosphate-modified carbon quantum dots. Microchim Acta 186, 736 (2019). https://doi.org/10.1007/s00604-019-3872-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3872-0