Abstract
Fluorescent polymer dots (PDs) with maximum excitation/emission wavelengths of 410/515 nm were prepared in water solution from 1,4-benzoquinone and ethylenediamine. The green fluorescence of these PDs is screened off by the red-colored oxidation product (PPDox, maximum absorption at 510 nm) formed by horseradish peroxidase (HRP)-catalyzed oxidation of p-phenylenediamine (PPD). It causes the reduction of the fluorescence intensity of the PDs due to spectral overlap and an inner filter effect (IFE). If glucose is enzymatically oxidized under the formation of H2O2, the formed H2O2 can be quantified by the above IFE. The assay for HRP activity and glucose have detection limits of 0.2 U·L−1 and 0.1 μM, respectively. The nanoprobe was further extended to an immunosorbent assay (ELISA) for the determination of insecticidal Cry1Ab/Ac protein with a detection limit of 0.25 ng·mL−1. The ELISA was applied to rice leaf analysis.

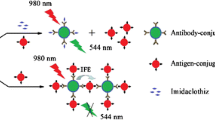
Schematic representation of fluorometrict enzyme-linked immunosorbent assay for Cry1Ab/Ac protein detection based on horseradish peroxidase (HRP)-triggered fluorescence quenching of polymer dots (PDs). Quenching is caused by an inner filter effect (IFE) caused by PPDox, the oxidation product of p-phenylenediamine (PPD).






Similar content being viewed by others
References
Veitch NC (2004) Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 65(3):249–259
Satvekar RK, Rohiwal SS, Raut AV, Karande VA, Tiwale BM, Pawar SH (2014) A silica-dextran nanocomposite as a novel matrix for immobilization of horseradish peroxidase, and its application to sensing hydrogen peroxide. Microchim Acta 181(1–2):71–77
Li DY, Ying YB, Wu J, Niessner R, Knopp D (2013) Comparison of monomeric and polymeric horseradish peroxidase as labels in competitive ELISA for small molecule detection. Microchim Acta 180(7–8):711–717
Cheng X, Huang Y, Yuan C, Dai K, Jiang H, Ma JM (2019) Colorimetric detection of α-glucosidase activity based on the etching of gold nanorods and its application to screen anti-diabetic drugs. Sensor Actuat B Chem 282:838–843
Tronstad C, Elvebakk O, Staal OM, Kalvøy H, Høgetveit JO, Jenssen TG, Birkeland KI, Martinsen ØG (2019) Non-invasive prediction of blood glucose trends during hypoglycemia. Anal Chim Acta 1052:37–48
Khan MI, Zhang Q, Wang YX, Saud S, Liu WW, Liu SR, Kong H, Wang CH, Uzzaman A, Xiao H, Fan LY, Cao CX (2019) Portable electrophoresis titration chip model for sensing of uric acid in urine and blood by moving reaction boundary. Sensor Actuat B Chem 286:9–15
Darabdhara G, Boruah PK, Das MR (2019) Colorimetric determination of glucose in solution and via the use of a paper strip by exploiting the peroxidase and oxidase mimicking activity of bimetallic cu-Pd nanoparticles deposited on reduced graphene oxide, graphitic carbon nitride, or MoS2 nanosheets. Microchim Acta 186(1):13
Liu JW, Luo Y, Wang YM, Duan LY, Jiang JH, Yu RQ (2016) Graphitic carbon nitride nanosheets-based ratiometric fluorescent probe for highly sensitive detection of H2O2 and glucose. ACS Appl Mater Inter 8(49):33439–33445
Karuppiah C, Palanisamy S, Chen SM, Veeramani V, Periakaruppan P (2014) Direct electrochemistry of glucose oxidase and sensing glucose using a screen-printed carbon electrode modified with graphite nanosheets and zinc oxide nanoparticles. Microchim Acta 181(15–16):1843–1850
Hovancová J, Šišoláková I, Oriňaková R, Oriňak A (2017) Nanomaterial-based electrochemical sensors for detection of glucose and insulin. J Solid State Electrochem 21(8):2147–2166
Parween S, Nahar P (2015) Femtogram detection of horseradish peroxidase by a common desktop scanner. J Biosci Bioeng 119(1):113–116
Huang S, Wang LM, Huang CS, Su W, Xiao Q (2016) Amino-functionalized graphene quantum dots based ratiometric fluorescent nanosensor for ultrasensitive and highly selective recognition of horseradish peroxidase. Sensor Actuat B-Chem 234:255–263
Wang Q, Xue R, Guo H, Wei Y, Yang W (2018) A facile horseradish peroxidase electrochemical biosensor with surface molecular imprinting based on polyaniline nanotubes. J Electroanal Chem 817:184–194
Wu JS, Liu WM, Ge JC, Zhang HY, Wang PF (2011) New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem Soc Rev 40(7):3483–3495
Ma JM, Cheng X, Peng FF, Zhang N, Li RF, Sun LH, Li ZL, Jiang H (2019) A polymer dots fluorescent sensor for detection of alkaline phosphatase activity and inhibitor evaluation. J Mater Sci 54:10055–10064
Sutariya PG, Soni H, Gandhi SA, Pandya A (2019) Novel luminescent paper based calix [4] arene chelation enhanced fluorescence-photoinduced electron transfer probe for Mn2+, Cr3+ and F. J Lumin 208:6–17
Liu SY, Wang H, He T, Qi L, Zhang ZQ (2016) Sensitive fluorimetric assays for α-glucosidase activity and inhibitor screening based on β-cyclodextrin-coated quantum dots. Luminescence 31(1):96–101
Chen S, Yu YL, Wang JH (2018) Inner filter effect-based fluorescent sensing systems: A review. Anal Chim Acta 999:13–26
Zhang J, Zhou R, Tang D, Hou X, Wu P (2018) Optically-active nanocrystals for inner filter effect-based fluorescence sensing: Achieving better spectral overlap. TrAC Trend Anal Chem 110:183–190
Wang T, Zeng LH, Li DL (2017) A review on the methods for correcting the fluorescence inner-filter effect of fluorescence spectrum. Appl Spectrosc Rev 52(10):883–908
Zhu SJ, Song YB, Shao JR, Zhao XH, Yang B (2015) Non-conjugated polymer dots with crosslink-enhanced emission in the absence of fluorophore units. Angew Chem Int Ed 54(49):14626–14637
Nasirian V, Chabok A, Barati A, Rafienia M, Arabi MS, Shamsipur M (2017) Ultrasensitive aflatoxin B1 assay based on FRET from aptamer labelled fluorescent polymer dots to silver nanoparticles labeled with complementary DNA. Microchim Acta 184(12):4655–4662
Liu MM, Huang R, Weisman A, Yu XY, Lee SH, Chen YL, Huang C, Hu SH, Chen XH, Tan WF, Liu F, Chen H, Shea KJ (2018) Synthetic polymer affinity ligand for bacillus thuringiensis (Bt) Cry1Ab/ac protein: The use of biomimicry based on the Bt protein–insect receptor binding mechanism. J Am Chem Soc 140(22):6853–6864
Wang X, Chen X, Xu J, Dai C, Shen W (2015) Degradation and detection of transgenic Bacillus thuringiensis DNA and proteins in flour of three genetically modified rice events submitted to a set of thermal processes. Food Chem Toxicol 84:89–98
Jiang Y, Ling L, Zhang L, Wang K, Li X, Cai M, Zhan M, Li C, Wang J, Cao C (2018) Comparison of transgenic Bt rice and their non-Bt counterpart in yield and physiological response to drought stress. Field Crops Res 217:45–52
Zhu M, Li M, Li G, Zhou Z, Liu H, Lei H, Shen Y, Wan Y (2015) Nanobody-based electrochemical immunoassay for bacillus thuringiensis Cry1Ab toxin by detecting the enzymatic formation of polyaniline. Microchim Acta 182:2451–2459
Cheng X, Huang Y, Li DY, Yuan C, Li ZL, Sun LH, Jiang H, Ma JM (2019) A sensitive polymer dots fluorescent sensor for determination of α-L-fucosidase activity in human serum. Sensor Actuators B Chem 288:38–43
Kong WH, Wu D, Xia L, Chen XF, Li GL, Qiu NN, Chen G, Sun ZW, You JM, Wu YN (2017) Carbon dots for fluorescent detection of α-glucosidase activity using enzyme activated inner filter effect and its application to anti-diabetic drug discovery. Anal Chim Acta 973:91–99
Li GL, Kong WH, Zhao M, Lu SM, Gong PW, Chen G, Xia L, Wang H, You JM, Wu YN (2016) A fluorescence resonance energy transfer (FRET) based "turn-on" nanofluorescence sensor using a nitrogen-doped carbon dot-hexagonal cobalt oxyhydroxide nanosheet architecture and application to alpha-glucosidase inhibitor screening. Biosens Bioelectron 79:728–735
Zhang MW, Cao XY, Li HK, Guan FR, Guo JJ, Shen F, Luo YL, Sun CY, Zhang LG (2012) Sensitive fluorescent detection of melamine in raw milk based on the inner filter effect of au nanoparticles on the fluorescence of CdTe quantum dots. Food Chem 135:1894–1900
Liu HJ, Li M, Xia YN, Ren XQ (2017) A turn-on fluorescent sensor for selective and sensitive detection of alkaline phosphatase activity with gold nanoclusters based on inner filter effect. ACS Appl Mate Inter 9:120–126
Acknowledgments
This study was funded by the Fundamental Research Funds for the Central Universities, China (2662018JC011 and 2662019PY023).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1852 kb)
Rights and permissions
About this article
Cite this article
Cheng, X., Sun, L., Li, R. et al. Organic polymer dot-based fluorometric determination of the activity of horseradish peroxidase and of the concentrations of glucose and the insecticidal protein toxin Cry1Ab/Ac. Microchim Acta 186, 731 (2019). https://doi.org/10.1007/s00604-019-3831-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3831-9