Abstract
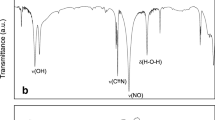
A protocol is described for chemical modification of graphene oxide with a Schiff base derived from diethylenetriamine and 2-hydroxy-4-methoxybenzophenone. The base was grafted onto an indium tin oxide (ITO) film and applied to electroanalytical determination of arsenite. Successful grafting was confirmed by Fourier transform-infrared spectroscopy, spectrophotometry, field emission scanning electron microscopy and cyclic voltammetry. Secondly, the coated ITO film served as a working electrode for the stripping voltammetric determination of arsenite. The analytical signal is generated by selective oxidation of metal species via multi-donor sites present in the derivatized Schiff base. The electroanalytical protocol was optimized by investigating the effects of deposition time, working potential, frequency and amplitude of square wave anodic stripping voltammetry. The method has attractive features including (a) the usage of a non-metallic, non-toxic and cost-effective material; (b) improved sensitivity (with limit of detection as low as 156 pM) due to better adsorption of arsenite in the Schiff base pockets on the ITO, and (c) the application to the determination of arsenite in real samples.

Schematic representation of the fabrication of a Schiff base-functionalized graphene oxide on an indium tin oxide (SB@SiO2@GO@ITO) electrode for selective electrochemical sensing of arsenite due to adsorption on multi-donor sites.







Similar content being viewed by others
References
Sattar A, Xie S, Hafeez MA, Wang X, Hussain HI, Iqbal Z, Pan Y, Iqbal M, Shabbir MA, Yuan Z (2016) Metabolism and toxicity of arsenicals in mammals. Environ Toxicol Pharmacol 48:214–224
Shakoor MB, Nawaz R, Husaain F, Raza M, Ali S, Rizwan M, Oh S-E, Ahmad S (2017) Human health implications, risk assessment and remediation of As-contaminated water: a critical review. Sci Total Environ 601:756–769
Sarkar A, Paul B (2016) The global menace of arsenic and its conventional remediation- a critical review. Chemosphere 158:37–49
Kabir ER, Rahman MS, Rahman I (2015) A review on endocrine disruptors and their possible impacts on human health. Environ Toxicol Pharmacol 40:241–258
Nguyen MH, Pham TD, Nguyen TL (2018) Speciation analysis of arsenic compounds by HPLC-ICP-MS: application for human serum and urine. J Anal Methods Chem 2018:9462019:1–8. https://doi.org/10.1155/2018/9462019
Luong JHT, Lam E, Male KB (2014) Recent advances in electrochemical detection of arsenic in drinking and ground waters. Anal Methods 6:6157–6169
Ma J, Sengupta MK, Yuan D, Dasgupta PK (2014) Speciation and detection of arsenic in aqueous samples: a review of recent progress in non-aromatic spectrometric methods. Anal Chim Acta 831:1–23
Rajkumar M, Thiagarajan S, Chen S-M (2011) Electrochemical detection of arsenic in various water samples. Int J Electrochem Sci 6:3164–3177
Zhou S, Han X, Fan H, Liu Y (2016) Electrochemical sensing toward trace As(III) based on mesoporous MnFe2O4/Au hybrid nanospheres modified glass carbon electrode. Sensors 16:935
Shah A, Zahid A, Khan A, Iftikhar FJ, Nisar J, Fernandez C, Akhter MS, Almutawah AA, Kraatz H-B (2019) Development of a highly sensitive electrochemical sensing platform for the trace level detection of lead ions. J Electrochem Soc 166:B3136–B3142
Anastasiadou ZD, Sipaki I, Jannakoudakis PD, Girousi ST (2011) Square-Wave Anodic Stripping Voltammetry (SWASV) for the determination of ecotoxic metals using a bismuth-film electrode. Anal Lett 44:761–777
Trachioti MG, Karantzalis AE, Harbac J, Prodromidis MI (2019) Low cost screen printed sensors on demand: instantly prepared sparked gold nanoparticles from Au/Si alloy for the determination of arsenic at the sub-ppb level. Sensors Actuators B Chem 281:273–280
Pungjunun K, Chaiyo S, Jantrahong I, Nantaphol S, Siangproh W, Chailapakul O (2018) Anodic stripping voltammetric determination of total arsenic using a gold nanoparticle-modified boron-doped diamond electrode on a paper-based device. Microchim Acta 185:324–331
Yang M, Chen X, Jiang TJ, Guo Z, Liu JH, Huang XJ (2016) Electrochemical detection of trace arsenic (III) by nanocomposite of nanorod-like α-MnO2 decorated with ∼5 nm Au nanoparticles: considering the change of arsenic speciation. Anal Chem 88:9720–9728
Cui L, Wu J, Ju H (2016) Label-free signal-on aptasensor for sensitive electrochemical detection of arsenite. Biosens Bioelectron 79:861–865
Wen S, Zhang C, Liang R, Chi B, Yuan Y, Qiu J (2017) Highly sensitive voltammetric determination of arsenite by exploiting arsenite-induced conformational change of ssDNA and the electrochemical indicator Methylene Blue. Microchim Acta 184:4047–4054
Sanghavi BJ, Gadhari NS, Kalambate PK, Karna SP, Srivastava AK (2015) Potentiometric stripping analysis of arsenic using a graphene paste electrode modified with a thiacrown ether and gold nanoparticles. Microchim Acta 182:1473–1481
Gao C, Yu X-Y, Xiong S-Q, Liu J-H, Huang X-J (2013) Electrochemical detection of arsenic(III) completely free from noble metal: Fe3O4 microspheres-room temperature ionic liquid composite showing better performance than gold. Anal Chem 85:2673–2680
Zhao Z, Li C, Wu H (2019) Reduced graphene oxide nanosheets modified with plasmonic gold-based hybrid nanostructures and with magnetite (Fe3O4) nanoparticles for cyclic voltammetric determination of arsenic(III). Microchim Acta 186:226–233
Cheng H, Zhang W, Wang Y, Liu J (2018) Graphene oxide as a stationary phase for speciation of inorganic and organic species of mercury, arsenic and selenium using HPLC with ICP-MS detection. Microchim Acta 185:9
Ahmad H, Umar K, Ali SG, Singh P, Islam SS, Khan HM (2018) Preconcentration and speciation of arsenic by using a graphene oxide nanoconstruct functionalized with a hyperbranched polyethyleneimine. Microchim Acta 185:290–297
Mutneja R, Srivastav N, Singh R, Kaur V, Bette N, Klemm V, Rafaja D, Wagler J, Kroke E (2019) Exploring superiority of silatranyl moiety as anchoring unit over its trialkoxysilyl analogue for covalent grafting via fabrication of functionalized mesoporous silica possessing azomethinic pincers for dye adsorption. Microporous Mesoporous Mater 273:265–272
Huang J-F, Chen H-H (2013) Gold-nanoparticle-embedded nafion composite modified on glassy carbon electrode for highly selective detection of arsenic(III). Talanta 116:852–859
Zhang W, Li B (2018) Influence of electrodeposition conditions on the microstructure and hardness of Ni-B/SiC nanocomposite coatings. Int J Electrochem Sci 13:3486–3500
Kaur P, Singh R, Kaur V, Talwar D (2018) Reusable Schiff base functionalized silica as a multi-purpose nanoprobe for fluorogenic recognition, quantification and extraction of Zn2+ ions. Sensors Actuators B Chem 254:533–541
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339–1339
Zhang N, Qiu H, Si Y, Wang W, Gao J (2011) Fabrication of highly porous biodegradable monoliths strengthened by graphene oxide and their adsorption of metal ions. CARBON 49:827–837
Rana S, Kaur R, Jain R, Prabhakar N (2018) Ionic liquid assisted growth of poly(3,4-ethylenedioxythiophene)/reduced graphene oxide based electrode: an improved electro-catalytic performance for the detection of organophosphorus pesticides in beverages. Arab J Chem In press. https://doi.org/10.1016/j.arabjc.2018.08.008
Gu T, Bu L, Huang Z, Liu Y, Tang Z, Liu Y, Huang S, Xie Q, Yao S, Tu X, Luo X, Luo S (2013) Dual-signal anodic stripping voltammetric determination of trace arsenic(III) at a glassy carbon electrode modified with internal-electrolysis deposited gold nanoparticles. Electrochem Commun 33:43–46
Han B, Pan M, Zhou J, Wang Y, Wang Z, Jiao J, Zhang C, Chen Q (2018) Facile synthesis of β-lactoglobulin-functionalized reduced graphene oxide and trimetallic PtAuPd nanocomposite for electrochemical sensing. Nanomaterials 8:724
Yang X, Wang F, Hu S (2006) The electrochemical oxidation of troxerutin and its sensitive determination in pharmaceutical dosage forms at PVP modified carbon paste electrode. Colloids Surf B 52:8–13
Mafa JP, Mabuba N, Arotiba OA (2016) An exfoliated graphite based electrochemical sensor for As(III) in water. Electroanalysis 28:1462–1469
Lopaz JR, Videa M (2012) Study of the ion transfer of quaternary ammonium ions by SWV. J Mex Chem Soc 56:417–425
Acknowledgments
The authors are thankful to CSIR, New Delhi for providing financial support (No. 01(2909)/17/EMR-II dated 03-05-2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 762 kb)
Rights and permissions
About this article
Cite this article
Kaur, R., Rana, S., Singh, R. et al. A Schiff base modified graphene oxide film for anodic stripping voltammetric determination of arsenite. Microchim Acta 186, 741 (2019). https://doi.org/10.1007/s00604-019-3807-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3807-9