Abstract
An electrochemical sensor is described for the simultaneous determination of hydroquinone (HQ) and catechol (CC) based on a nanocomposite consisting of gold nanoparticles and graphitic carbon nitride (g-C3N4). The nanocomposite was synthesized via one-step thermal polymerization route and characterized by X-ray diffraction, transmission electron microscopy, and Fourier transform infrared techniques. The results confirmed the close contact between gold nanoparticles and g-C3N4. The nanocomposites exhibited the enhanced electrocatalytic redox towards HQ and CC. A glassy carbon electrode was modified with the nanocomposite to obtain a sensor that exhibited favorable analytical properties in the simultaneous detection of HQ and CC, with voltammetric peaks typically near −0.14 and − 0.02 V (vs. saturated calomel electrode). Linear responses are found between 1.0 and 320 μM for HQ (with a 0.3 μM detection limit; at S/N = 3), and between 0.1 and 320 μM for CC (with a 0.04 μM detection limit; at S/N = 3). The sensor was applied for the simultaneous determination of HQ and CC in spiked water samples, and acceptable recoveries were achieved. The superior sensing properties of the electrode are attributed to the synergy between the microstructure (heterojunction and porosity) and the π interactions between phenolic isomers and g-C3N4.

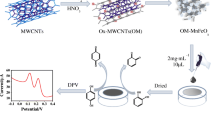
A novel electrochemical sensor is demonstrated for the simultaneous determination of hydroquinone and catechol based on a nanocomposite consisting of gold nanoparticles (AuNPs) and graphitic carbon nitride (g-C3N4).






Similar content being viewed by others
References
Huang KJ, Wang L, Liu YJ, Gan T, Liu YM, Wang LL, Fan Y (2013) Synthesis and electrochemical performances of layered tungsten sulfide-graphene nanocomposite as a sensing platform for catechol, resorcinol and hydroquinone. Electrochim Acta 10:379–387. https://doi.org/10.1016/j.electacta.2013.06.060
Yuan X, Yuan D, Zeng F, Zou W, Tzorbatzogloub F, Tsiakaras P, Wang Y (2013) Preparation of graphitic mesoporous carbon for the simultaneous detection of hydroquinone and catechol. Appl Catal B Environ 129:367–374. https://doi.org/10.1016/j.apcatb.2012.09.017
Yang X, Kirsch J, Fergus J, Simonian A (2013) Modeling analysis of electrode fouling during electrolysis of phenolic compounds. Electrochim Acta 94:259–268. https://doi.org/10.1016/j.electacta.2013.01.019
Sun W, Wang YH, Lu YX, Hu AH, Shi F, Sun ZF (2013) High sensitive simultaneously electrochemical detection of hydroquinone and catechol with a poly (crystal violet) functionalized graphene modified carbon ionic liquid electrode. Sens Actuators B: Chem 188:564–570. https://doi.org/10.1016/j.snb.2013.07.032
Lourenco ELB, Ferreira A, Pinto E, Yonamine M, Farsky SHP (2006) On-Fiber derivatization of SPME extracts of phenol, hydroquinone and catechol with GC-MS detection. Chromatographia 63:175–179. https://doi.org/10.1365/s10337-006-0719-80,009-5893/06/02
Marrubini G, Calleri E, Coccini T, Castoldi AF, Manzo L (2015) Direct analysis of phenol, catechol and hydroquinone in human urine by coupled-column HPLC with fluorimetric detection. Chromatographia 62:25–31. https://doi.org/10.1365/s10337-005-0570-30,009-5893/05/07
Song YW, Zhao MM, Li H, Wang XT, Cheng YF, Ding LJ, Fan S, Chen SG (2018) Facile preparation of urchin-like NiCo2O4 microspheres as oxidase mimetic for colormetric assay of hydroquinone. Sens Actuators B: Chem 255:1927–1936. https://doi.org/10.1016/j.snb.2017.08.204
Zhao LJ, Lv BQ, Yuan HY, Zhou ZD, Xiao D (2017) A sensitive chemiluminescence method for determination of hydroquinone and catechol. Sensors 7:578–588. https://doi.org/10.3390/s7040578
Liu LY, Ma Z, Zhu XH, Alshahrani LA, Tie SL, Nan JM (2016) A glassy carbon electrode modified with carbon nano-fragments and bismuth oxide for electrochemical analysis of trace catechol in the presence of high concentrations of hydroquinone. Microchim Acta 183:3293–3301. https://doi.org/10.1007/s00604-016-1973-6
Nasr B, Abdellatif G, Cańizares P, Sáez C, Lobato J, Rodrigo MA (2015) Electrochemical oxidation of hydroquinone, resorcinol, and catechol on boron-doped diamond anodes. Environ Sci Technol 39:7234–7239. https://doi.org/10.1021/es0500660
Coroş M, Pogăcean F, Măgeruşan L, Roşu MC, Porav AS, Socaci C, Bende A, Staden RISV, Pruneanu S (2018) Graphene-porphyrin composite synthesis through graphite exfoliation: the electrochemical sensing of catechol. Sens Actuators B: Chem 256:665–673. https://doi.org/10.1016/j.snb.2017.09.205
Riskin M, Vered RT, Bourenko T, Granot E, Willner I (2008) Imprinting of molecular recognition sites through electropolymerization of functionalized Au nanoparticles: development of an electrochemical TNT sensor based on π-donor−acceptor interactions. J Am Chem Soc 130:9726–9733. https://doi.org/10.1021/ja711278c
Ong WJ, Tan LL, Ng YH, Yong ST, Chai SP (2016) Graphitic carbon nitride (g-C3N4)-based photocatalytsts for artificial photosynthesis and environmental remediation: are we a step closer to achieving sustainablility? Chem Rev. 116:7159–7329. https://doi.org/10.1021/acs.chemrev.6b00075
Sun YP, Ha W, Chen J, Qi HY, Shi YP (2016) Advances and applications of graphitic carbon nitride as sorbent in analytical chemistry for sample pretreatment: A review. TrAC Trends Anal Chem 84:12–21. https://doi.org/10.1016/j.trac.2016.03.002
Wu JJ, Li N, Fang HB, Li XT, Zheng YZ, Tao X (2019) Nitrogen vacancies modified graphitic carbon nitride: Scalable and one-step fabrication with efficient visible-light-driven hydrogen evolution. Chem Eng J 358:20–29. https://doi.org/10.1016/j.cej.2018.09.208
Wang XC, Maeda K, Thomas A, Takanabe K, Xin G, Carlsson JM, Domen K, Antonietti M (2008) A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat Mater 8:76–80. https://doi.org/10.1038/nmat2317
Huang DL, Li ZH, Zeng GM, Zhou CY, Xue WJ, Gong XM, Yan XL, Chen S, Wang WJ, Cheng M (2019) Megamerger in photocatalytic field: 2D g-C3N4 nanosheets serve as support of 0D nanomaterials for improving photocatalytic performance. Appl Catal B: Environ 240:153–173. https://doi.org/10.1016/j.apcatb.2018.08.071
Masih D, Ma YY, Rohani S (2017) Graphitic C3N4 based noble-metal-free photocatalyst systems: A review. Appl Catal B: Environ 206:556–588. https://doi.org/10.1016/j.apcatb.2017.01.061
Zhang JT, Tang Y, Lee K, Ouyang M (2010) Nonepitaxial growth of hybrid core-shell nanostructures with large lattice mismatches. Science 327:1634–1638. https://doi.org/10.1126/science.1184769
Mamba G, Mishra AK (2016) Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl Catal B: Environ 198:347–377. https://doi.org/10.1016/j.apcatb.2016.05.052
Zheng Y, Jiao Y, Zhu YH, Cai QR, Vasileff A, Li LH, Chen Y, Qiao SZ (2017) Molecule-level g-C3N4 coordinated transition metals as a new class of electrocatalysts for oxygen electrode reactions. J Am Chem Soc 139:3336–3339. https://doi.org/10.1021/jacs.6b13100
Jin J, Fu X, Liu Q, Zhang J (2013) A highly active and stable electrocatalyst for oxygen reduction reaction based on a grapheme-supported g-C3N4@cobalt oxide core-shell hybrid in alkaline solution. J Mater Chem A 1:10538–10,545. https://doi.org/10.1039/c3ta11144j
Guo H, Su Y, Shen YL, Long YM, Li WF (2019) In situ decoration of Au nanoparticles on carbon nitride using a single-source precursor and its application for the detection of tetracycline. J Colloid Interf Sci 536:646–654. https://doi.org/10.1016/j.jcis.2018.10.104
Ran JR, Jaroniec M, Qiao SZ (2018) Cocatalysts in semiconductor-based photocatalytic CO2 reduction: achievements, challenges, and opportunities. Adv Mater 30:201704649. https://doi.org/10.1002/adma.201704649
Xu L, Ling SY, Li HN, Yan PC, Xia JX, Qiu JX, Li HM, Yuan SQ (2017) Photoelectrochemical monitoring of 4-chlorophenol by plasmonic Au/graphitic carbon nitride composites. Sens Actuators B: Chem 240:308–314. https://doi.org/10.1016/j.snb.2016.08.038
Li HL, Gao Y, Xiong Z, Liao C, Shih K (2018) Enhanced selective photocatalytic reduction of CO2 to CH4 over plasmonic Au modified g-C3N4 photocatalyst under UV–vis light irradiation. Appl Surf Sci 439:552–559. https://doi.org/10.1016/j.apsusc.2018.01.071
Mircescu NE, Oltean M, Chis V, Leopold N (2012) FTIR, FT-Raman, SERS and DFT study on melamine. Vibrational Spectroscopy 62:165–171. https://doi.org/10.1016/j.vibspec.2012.04.008
Fu YS, Huang T, Jia BQ, Zhu JW, Wang X (2017) Reduction of nitrophenols to aminophenols under concerted catalysis by Au/g-C3N4 contact system. Appl Catal B: Environ 202:430–437. https://doi.org/10.1016/j.apcatb.2016.09.051
Rounaghi SA, Vanpouche DEP, Eshghi H, Scudino S, Esmaeili E, Oswald S, Eckert J (2017) A combined experimental and theoretical investigation of the Al-Melamine reactive milling system: A mechanistic study towards AlN-based ceramics. J Alloy Compd 729:240–248. https://doi.org/10.1016/j.jallcom.2017.09.168
Zhao H, Ding XL, Zhang B, Li YX, Wang CY (2017) Enhanced photocatalytic hydrogen evolution along with byproducts suppressing over Z-scheme CdxZn1-xS/Au/g-C3N4 photocatalysts under visible light. Sci Bull 62:602–609. https://doi.org/10.1016/j.scib.2017.03.005
Niu XH, Yang X, Mo ZL, Liu NJ, Guo RB, Pan Z, Liu ZY (2019) Electrochemical chiral sensing of tryptophan enantiomers by using 3D nitrogen-doped reduced grapheme oxide. Microchem Acta 186:557–2365. https://doi.org/10.1007/s00604-019-3682-4
Wang Y, Yao J, Li HR, Su DS, Antonietti M (2011) Highly selective hydrogenation of phenol and derivatives over Pd@Carbon nitride catalyst in aqueous media. J Am Chem Soc 133:2362–2365. https://doi.org/10.1021/ja109856y
Acknowledgements
This work is supported by the National Natural Science Foundation of China (21005053), the Priority Academic Program Development of Jiangsu Higher Education Institutions and the Project of Scientific and Technologic Infrastructure of Suzhou (SZS201708).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 1.12 mb)
Rights and permissions
About this article
Cite this article
Guo, H., Shen, Y., Ouyang, H. et al. A voltammetric sensor for simultaneous determination of hydroquinone and catechol by using a heterojunction prepared from gold nanoparticle and graphitic carbon nitride. Microchim Acta 186, 819 (2019). https://doi.org/10.1007/s00604-019-3798-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3798-6