Abstract
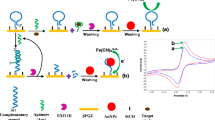
A rapid method for the sensitive detection of phenytoin (PHT) by branched gold nanoparticles (B-AuNPs) is described. These nanoparticles were synthesized by adding methanol as the reducing agent and poly(ethylene glycol) as the stabilizer at 70 °C. The B-AuNPs are red in color with an absorption maximum at 540 nm when prepared in situ. However, the color becomes increasingly weaker when PHT is present in increasing concentrations. This method can determine PHT over the 67–670 ng·mL−1 concentration range, with detection limit of 21 ng·mL−1. The relative standard deviation for five replicate measurements at 68 and 530 ng·mL−1 of PHT was 3.2% and 1.2%, respectively. The method was applied to the determination of PHT in plasma samples of epileptic patients, and the results were in agreement with those obtained by a standard official method.

Branched gold nanoparticles (AuNPs) prepared in situ have a red color with an absorption maximum at 540 nm. The color becomes increasingly weaker with decreasing the intensity of the characteristic SPR band when PHT is present in increasing concentration. The current assay is capable of determining PHT over the 67–670 ng·mL−1 concentration range with a limit of detection of 21 ng·mL−1.


Similar content being viewed by others
References
von Winckelmann SL, Spriet I, Willems L (2008) Therapeutic drug monitoring of phenytoin in critically ill patients. Pharmacotherapy 28:1391–1400
Soldin SJ, Wang E, Verjee Z, Elin RJ (2003) Phenytoin overview--metabolite interference in some immunoassays could be clinically important. Arch Pathol Lab Med 127:1623–1625
Nelson MH, Birnbaum AK, Nyhus PJ, Remmel RP (1998) A capillary GC-MS method for analysis of phenytoin and [13C3]-phenytoin from plasma obtained from pulse dose pharmacokinetic studies1. J Pharm Biomed Anal 17:1311–1323
Qu L, Fan Y, Wang W, Ma K, Yin Z (2016) Development, validation and clinical application of an online-SPE-LC-HRMS/MS for simultaneous quantification of phenobarbital, phenytoin, carbamazepine, and its active metabolite carbamazepine 10,11-epoxide. Talanta 158:77–88
Anilanmert B, Çavuş F, Narin I, Cengiz S, Sertler Ş, Özdemir AA, Açikkol M (2016) Simultaneous analysis method for GHB, ketamine, norketamine, phenobarbital, thiopental, zolpidem, zopiclone and phenytoin in urine, using C18 poroshell column. J Chromatogr B 1022:230–241
Lin P-C, Hsieh Y-H, Liao F-F, Chen S-H (2010) Determination of free and total levels of phenytoin in human plasma from patients with epilepsy by MEKC: an adequate alternative to HPLC. Electrophoresis 31(9):1572–1582
Serralheiro A, Alves G, Fortuna A, Rocha M, Falcão A (2013) First HPLC–UV method for rapid and simultaneous quantification of phenobarbital, primidone, phenytoin, carbamazepine, carbamazepine-10,11-epoxide, 10,11-trans-dihydroxy-10,11-dihydrocarbamazepine, lamotrigine, oxcarbazepine and licarbazepine in human plasma. J Chromatogr B 925:1–9
Guan F, Uboh CE, Soma LR, Birks EK, Teleis D, Rudy JA, Watson AO, Tsang DS (2000) Quantification of phenytoin and its metabolites in equine plasma and urine using high-performance liquid chromatography. J Chromatogr B Biomed Sci App 746:209–218
Hara S, Hagiwara J, Fukuzawa M, Ono N, Kuroda T (1999) Determination of phenytoin and its major metabolites in human serum by high-performance liquid chromatography with fluorescence detection. Anal Sci 15:371–375
Dean KJ, Thompson SG, Burd JF, Buckler RT (1983) Simultaneous determination of phenytoin and phenobarbital in serum or plasma by substrate-labeled fluorescent immunoassay. Clin Chem 29:1051–1056
Aman T, Firdous S, Khan IU, Kazi AA (2001) Spectrophotometric determination of phenytoin sodium in pure and pharmaceutical preparations. Microchim Acta 137:121–126
Korany M (1998) Extraction-spectrophotometric determination of phenytoin in capsules and plasma using potassium permanganate/dicyclohexano-24-crown-8. Talanta 46:9–14
Mantri MA, Shanmukhappa S (2010) UV spectrophotometric estimation of phenytoin sodium in pure and pharmaceutical formulations. Asian J Chem 22:2447–2449
Saleh GA (1998) Charge-transfer complexes of barbiturates and phenytoin. Talanta 46:111–121
Jaffery NF, Ahmad SN, Jailkhani BL (1983) A spectrophotometric method for simultaneous estimation of phenytoin and carbamazepine. J Pharmacol Methods 9:33–39
Wallace J, Biggs J, Dahl EV (1965) Determination of diphenylhydantoin by ultraviolet spectrophotometry. Anal Chem 37:410–413
Stanishevsky AV, Williamson H, Yockell-Lelievre H, Rast L, Ritcey AM (2006) Synthesis of complex shape gold nanoparticles in water–methanol mixtures. J Nanosci Nanotechnol 6:2013–2017
Jazayeri MH, Aghaie T, Avan A, Vatankhah A, Ghaffari MRS (2018) Colorimetric detection based on gold nano particles (GNPs): an easy, fast, inexpensive, low-cost and short time method in detection of analytes (protein, DNA, and ion). Sens Bio-Sens Res 20:1–8
Vasimalai N, John SA (2013) Aggregation and de-aggregation of gold nanoparticles induced by polyionic drugs: spectrofluorimetric determination of picogram amounts of protamine and heparin drugs in the presence of 1000-fold concentration of major interferences. J Mater Chem B 1:5620
Shawky SM, Awad AM, Allam W, Alkordi MH, EL-Khamisy SF (2017) Gold aggregating gold: a novel nanoparticle biosensor approach for the direct quantification of hepatitis C virus RNA in clinical samples. Biosens Bioelectron 92:349–356
Rastegarzadeh S, Hashemi F (2014) A surface plasmon resonance sensing method for determining captopril based on in situ formation of silver nanoparticles using ascorbic acid. Spectrochim Acta A Mol Biomol Spectrosc 122:536–541
Karami-Bonari AR, Tamizi E, Samini M, Jabbaribar F, Jouyban A (2009) HPLC method for simultaneous determination of five antiepileptic drugs in rat plasma. Asian J Chem 21:6501–6507
Borkowska Z, Tymosiak-Zielinska A, Nowakowski R (2004) High catalytic activity of chemically activated gold electrodes towards electro-oxidation of methanol. Electrochim Acta 49:2613–2621
Hernández J, Solla-Gullón J, Herrero E, Aldaz A, Feliu JM (2006) Methanol oxidation on gold nanoparticles in alkaline media: unusual electrocatalytic activity. Electrochim Acta 52:1662–1669
Jena BK, Raj CR (2007) Synthesis of flower-like gold nanoparticles and their Electrocatalytic activity towards the oxidation of methanol and the reduction of oxygen. Langmuir 23:4064–4070
Manson J, Kumar D, Meenan BJ, Dixon D (2011) Polyethylene glycol functionalized gold nanoparticles: the influence of capping density on stability in various media. Gold Bull 44:99–105
Oyaizu K, Ohtani Y, Shiozawa A, Sugawara K, Saito T, Yuasa M (2005) Highly stable gold(III) complex with a hydantoin ligand in alkaline media. Inorg Chem 44:6915–6917
Corbierre MK, Lennox RB (2005) Preparation of thiol-capped gold nanoparticles by chemical reduction of soluble au(I)−thiolates. Chem Mater 17:5691–5696
Ingle JD, Crouch SR (1988) Spectrochemical analysis. Prentice Hall, Englewood Cliffs
Krasowski MD, Penrod LE (2012) Clinical decision support of therapeutic drug monitoring of phenytoin: measured versus adjusted phenytoin plasma concentrations. BMC Med Inform Decis Mak 12:7. https://doi.org/10.1186/1472-6947-12-7
Lockard JS, Levy RH, Uhlir V, Farquhar JS (1976) Interactions of phenytoin and phenobarbital in terms of order and temporal spacing of administration in monkeys. Epilepsia 17:481–485
Acknowledgements
This work was financially supported by the “Vice Chancellor of Research and Technology, Tabriz University of Medical Sciences” under the grant number of 594252.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 105 kb)
Rights and permissions
About this article
Cite this article
Khoubnasabjafari, M., Samadi, A. & Jouyban, A. In-situ microscale spectrophotometric determination of phenytoin by using branched gold nanoparticles. Microchim Acta 186, 422 (2019). https://doi.org/10.1007/s00604-019-3546-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3546-y