Abstract
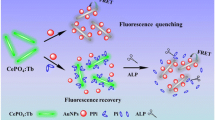
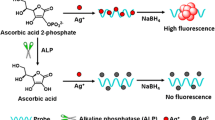
The authors describe a fluorometric and resonance Rayleigh scattering dual-mode scheme for detection of the activity and inhibition of alkaline phosphatase (ALP). The method utilizes (a) CoOOH nanoflakes, which have high resonance Rayleigh scattering activity and can strongly adsorb ssDNA, and (b) Co(II)-dependent DNAzyme assisted signal amplification. ALP specifically catalyzes the hydrolysis of ascorbic acid-2-phosphate to produce ascorbic acid which reduces CoOOH nanoflakes to Co(II) ion. The Co(II)-dependent DNAzyme is then activated by Co(II) ion, and this results in the cleavage of a substrate labeled with both a fluorophore and a quencher. Following hydrolysis, fluorophore and quencher become separated and the fluorescence measured at excitation/emission wavelengths of 490/518 nm recovers, while the RRS signal at 405 nm decreases. The method works on the 0.2 to 2000 U L−1 ALP activity range, and the detection limit is 0.05 U L−1. The method was used to validate the mechanism of the action of two classical ALP inhibitors (EDTA and Na3VO4). Conceivably, it can also be applied to screen for ALP inhibitors.

A bioprobe composed of cobalt oxyhydroxide (CoOOH) nanoflakes as the substrate and carrier, and Co2+-dependent DNAzyme containing guanine ribonucleotide (rG) and uracil ribonucleotide (rU) for circularly signal amplification is used for fluorometric determination of alkaline phosphatase (ALP) activity.





Similar content being viewed by others
References
Deng JJ, Yu P, Wang YX, Mao LQ (2015) Real-time Ratiometric fluorescent assay for alkaline phosphatase activity with stimulus responsive infinite coordination polymer nanoparticles. Anal Chem 87(5):3080–3086. https://doi.org/10.1021/ac504773n
Freeman R, Finder T, Gill R, Willner I (2010) Probing protein kinase (CK2) and alkaline phosphatase with CdSe / ZnS quantum dots. Nano Lett 10(6):2192–2196. https://doi.org/10.1021/nl101052f
Li CM, Zhen SJ, Wang J, Li YF, Huang CZ (2013) A gold nanoparticles-based colorimetric assay for alkaline phosphatase detection with tunable dynamic range. Biosens Bioelectron 43:366–371. https://doi.org/10.1016/j.bios.2012.12.015
Park J, Helal A, Kim HS, Kim Y (2012) Fluorogenic assay of alkaline phosphatase activity based on the modulation of excited-state intramolecular proton transfer. Bioorg Med Chem Lett 22(17):5541–5544. https://doi.org/10.1016/j.bmcl.2012.07.021
Dong L, Miao QQ, Hai ZJ, Yuan Y, Liang GL (2015) Enzymatic Hydrogelation-induced fluorescence turn-off for sensing alkaline phosphatase in vitro and in living cells. Anal Chem 87(13):6475–6478. https://doi.org/10.1021/acs.analchem.5b01657
Xiao T, Sun J, Zhao JH, Wang S, Liu GY, Yang XR (2018) FRET effect between fluorescent Polydopamine nanoparticles and MnO2 Nanosheets and its application for sensitive sensing of alkaline phosphatase. Acs Appl Mater Inter 10(7):6560–6569. https://doi.org/10.1021/acsami.7b18816
Liu SY, Wang XY, Pang S, Na WD, Yan X, Su XG (2014) Fluorescence detection of Adenosine-5′-triphosphate and alkaline phosphatase based on the generation of CdS quantum dots. Anal Chim Acta 827:103–110. https://doi.org/10.1016/j.aca.2014.04.027
Zheng FY, Guo SH, Zeng F, Li J, Wu SZ (2014) Ratiometric fluorescent probe for alkaline phosphatase based on betaine-modified Polyethylenimine via excimer/monomer conversion. Anal Chem 86(19):9873–9879. https://doi.org/10.1021/ac502500e
Wang HB, Li Y, Chen Y, Zhang ZP, Gan T, Liu YM (2018) Determination of the activity of alkaline phosphatase by using nanoclusters composed of flower-like cobalt Oxyhydroxide and copper nanoclusters as fluorescent probes. Microchim Acta 185(2):102. https://doi.org/10.1007/s00604-017-2622-4
Jiao HP, Chen J, Li WY, Wang FY, Zhou HP, Li YX, Yu C (2014) Nucleic acid-regulated Perylene probe-induced gold nanoparticle aggregation: a new strategy for colorimetric sensing of alkaline phosphatase activity and inhibitor screening. Acs Appl Mater Inter 6(3):1979–1985. https://doi.org/10.1021/am405020b
Shen CC, Li XZ, Rasooly A, Guo LY, Zhang KN, Yang MH (2016) A single electrochemical biosensor for detecting the activity and inhibition of both protein kinase and alkaline phosphatase based on phosphate ions induced deposition of redox precipitates. Biosens Bioelectron 85:220–225. https://doi.org/10.1016/j.bios.2016.05.025
Ran B, Zheng W, Dong M, Xianyu Y, Chen Y, Wu J, Qian Z, Jiang X (2018) Peptide-mediated controllable cross-linking of gold nanoparticles for immunoassays with tunable detection range. Anal Chem 90(13):8234–8240. https://doi.org/10.1021/acs.analchem.8b01760
Hai ZJ, Li JD, Wu JJ, Xu JC, Liang GL (2017) Alkaline phosphatase-triggered simultaneous Hydrogelation and Chemiluminescence. J Am Chem Soc 139(3):1041–1044. https://doi.org/10.1021/jacs.6b11041
Jiang H, Wang XM (2012) Alkaline phosphatase-responsive anodic Electrochemiluminescence of CdSe nanoparticles. Anal Chem 84(16):6986–6993. https://doi.org/10.1021/ac300983t
Zeng Y, Ren JQ, Wang SK, Mai JM, Qu B, Zhang Y, Shen AG, Hu JM (2017) Rapid and reliable detection of alkaline phosphatase by a hot spots amplification strategy based on well-controlled assembly on single nanoparticle. Acs Appl Mater Inter 9(35):29547–29553. https://doi.org/10.1021/acsami.7b09336
Liu SG, Li N, Han L, Li LJ, Li NB, Luo HQ (2018) Size-dependent modulation of fluorescence and light scattering: a new strategy for development of ratiometric sensing. Mater Horiz 5(3):454–460. https://doi.org/10.1039/c7mh00872d
Halawa MI, Gao WY, Saqib M, Kitte SA, Wu FX, Xu GB (2017) Sensitive detection of alkaline phosphatase by switching on gold nanoclusters fluorescence quenched by pyridoxal phosphate. Biosens Bioelectron 95:8–14. https://doi.org/10.1016/j.bios.2017.03.073
Ma JL, Yin BC, Wu X, Ye BC (2016) Copper-mediated DNA-Scaffolded silver nanocluster on-off switch for detection of pyrophosphate and alkaline phosphatase. Anal Chem 88(18):9219–9225. https://doi.org/10.1021/acs.analchem.6b02465
Knirsch KC, Berner NC, Nerl HC, Cucinotta CS, Gholamvand Z, McEvoy N, Wang ZX, Abramovic I, Vecera P, Halik M, Sanvito S, Duesberg GS, Nicolosi V, Hauke F, Hirsch A, Colernan JN, Backes C (2015) Basal-plane functionalization of chemically exfoliated molybdenum disulfide by Diazonium salts. ACS Nano 9(6):6018–6030. https://doi.org/10.1021/acsnano.5b00965
Wang QH, Kalantar-Zadeh K, Kis A, Coleman JN, Strano MS (2012) Electronics and optoelectronics of two-dimensional transition metal Dichalcogenides. Nat Nanotechnol 7(11):699–712. https://doi.org/10.1038/Nnano.2012.193
Xiao MS, Man TT, Zhu CF, Pei H, Shi JY, Li L, Qu XM, Shen XZ, Li J (2018) MoS2 Nanoprobe for MicroRNA quantification based on duplex-specific nuclease signal amplification. Acs Appl Mater Inter 10(9):7852–7858. https://doi.org/10.1021/acsami.7b18984
Cen Y, Yang Y, Yu RQ, Chen TT, Chu X (2016) A cobalt Oxyhydroxide Nanoflake-based Nanoprobe for the sensitive fluorescence detection of T4 polynucleotide kinase activity and inhibition. Nanoscale 8(15):8202–8209. https://doi.org/10.1039/c6nr01427e
Zhu CF, Zeng ZY, Li H, Li F, Fan CH, Zhang H (2013) Single-layer MoS2-based Nanoprobes for homogeneous detection of biomolecules. J Am Chem Soc 135(16):5998–6001. https://doi.org/10.1021/ja4019572
Li LB, Wang C, Liu KY, Wang YH, Liu K, Lin YQ (2015) Hexagonal cobalt Oxyhydroxide-carbon dots hybridized surface: high sensitive fluorescence turn-on probe for monitoring of ascorbic acid in rat brain following brain ischemia. Anal Chem 87(6):3404–3411. https://doi.org/10.1021/ac5046609
Li N, Zhu YD, Liu T, Liu SG, Lin SM, Shi Y, Luo HQ, Li NB (2017) Turn-on fluorescence detection of pyrophosphate anion based on DNA-attached cobalt Oxyhydroxide. New J Chem 41(5):1993–1996. https://doi.org/10.1039/c6nj03491h
Liu SF, Cheng CB, Gong HW, Wang L (2015) Programmable Mg2+-dependent DNAzyme switch by the catalytic hairpin DNA assembly for dual-signal amplification toward homogeneous analysis of protein and DNA. Chem Commun 51(34):7364–7367. https://doi.org/10.1039/c5cc01649e
Zou MQ, Li DX, Yuan R, Xiang Y (2017) Metal-ion dependent DNAzyme recycling amplification for sensitive and homogeneous Immuno-proximity binding assay of alpha-fetoprotein biomarker. Biosens Bioelectron 92:624–629. https://doi.org/10.1016/j.bios.2016.10.044
Huang JY, Zhao L, Lei W, Wen W, Wang YJ, Bao T, Xiong HY, Zhang XH, Wang SF (2018) A high-sensitivity electrochemical Aptasensor of carcinoembryonic antigen based on graphene quantum dots-ionic liquid-Nafion Nanomatrix and DNAzyme-assisted signal amplification strategy. Biosens Bioelectron 99:28–33. https://doi.org/10.1016/j.bios.2017.07.036
Jiang JJ, Lin XY, Diao GW (2017) Smart combination of Cyclodextrin polymer host guest recognition and Mg2+-assistant cyclic cleavage reaction for sensitive electrochemical assay of nucleic acids. Acs Appl Mater Inter 9(42):36688–36694. https://doi.org/10.1021/acsami.7b13132
Wen ZB, Liang WB, Zhuo Y, Xiong CY, Zheng YN, Yuan R, Chai YQ (2017) An efficient target-intermediate recycling amplification strategy for ultrasensitive fluorescence assay of intracellular Lead ions. Chem Commun 53(54):7525–7528. https://doi.org/10.1039/c7cc04104g
Li N, Li YL, Gao XN, Yu ZZ, Pan W, Tang B (2017) Multiplexed gene silencing in living cells and in vivo using a DNAzymes-CoOOH nanocomposite. Chem Commun 53(36):4962–4965. https://doi.org/10.1039/c7cc00822h
Meng HM, Zhang XB, Yang C, Kuai HL, Mao GJ, Gong L, Zhang WH, Feng SL, Chang JB (2016) Efficient two-photon fluorescence Nanoprobe for turn-on detection and imaging of ascorbic acid in living cells and tissues. Anal Chem 88(11):6057–6063. https://doi.org/10.1021/acs.analchem.6b01352
Li JY, Si L, Bao JC, Wang ZY, Dai ZH (2017) Fluorescence regulation of poly (thymine)-templated copper nanoparticles via an enzyme-triggered reaction toward sensitive and selective detection of alkaline phosphatase. Anal Chem 89(6):3681–3686. https://doi.org/10.1021/acs.analchem.6b05112
Curti C, Pizauro JM, Ciancaglini P, Leone FA (1987) Probing protein kinase (CK2) and alkaline phosphatase with CdSe/ZnS quantum dots. Nano Lett 10(6):2192–2196. https://doi.org/10.1021/nl101052f
Whisnant AR, Gilman SD (2002) Studies of reversible inhibition, irreversible inhibition, and activation of alkaline phosphatase by capillary electrophoresis. Anal Biochem 307(2):226–234. https://doi.org/10.1039/c7cc04104g
Acknowledgements
The authors sincerely acknowledge the support of the National Natural Science Foundation of China (No. 21675131, 21273174), and the Natural Science Foundation of Chongqing (No. CSTC-2015jcyjB50001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1251 kb)
Rights and permissions
About this article
Cite this article
Zhou, J., Ling, Y., Li, N.B. et al. Fluorometric and resonance Rayleigh scattering dual-mode bioprobe for determination of the activity of alkaline phosphatase based on the use of CoOOH nanoflakes and cobalt(II)-dependent DNAzyme-assisted amplification. Microchim Acta 186, 437 (2019). https://doi.org/10.1007/s00604-019-3528-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3528-0