Abstract
A black phosphorene (BPE) and poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT:PSS) hybrid was used for the immobilization of hemin on a carbon ionic liquid electrode (CILE). BPE inside the PEDOT:PSS film was stable without adverse effects of water and oxygen. The hemin-modified electrode facilitates electrochemical communication with a couple of well-shaped and enhanced redox waves. Therefore BPE exhibits an accelerating function to the electron movement. This sensor exhibits excellent electrocatalytic effects on the reduction of various substrates including trichloroacetic acid (TCA), nitrite and H2O2. As for TCA, the reduction current at −0.36 V (vs. Ag/AgCl) increases linearly in the concentration range from 2.0 to 180 mmol·L−1 with a detection limit of 0.67 mmol·L−1 (at 3σ). As for nitrite, the reduction current at −0.59 Vis linear in the 1.0 to 10.5 mmol·L−1 concentration range, and the detection limit is 0.33 mmol·L−1 (at 3σ). As for H2O2, the reduction current at −0.033 V (vs. Ag/AgCl) is linear in the concentration range from 4.0 to 35.0 mmol·L−1 and the detection limit is 1.3 mmol·L−1 (at 3σ). The real sample was analyzed with satisfactory results, which indicated that BPE had potential applications in the field of electrochemical biosensor.

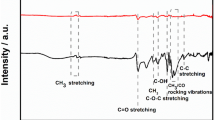
Photos of (a) black phosphorene (BPE) solution, (b) poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT:PSS), (c) BPE-PEDOT:PSS (1:5) dispersion, and the fabrication procedure of this electrochemical sensor. It was applied to the determination of trichloroacetic acid, nitrite and hydrogen peroxide.



Similar content being viewed by others

References
Wang H, Yang XZ, Shao W, Chen SC, Xie JF, Zhang XD, Wang J, Xie Y (2015) Ultrathin black phosphorus nanosheets for efficient singlet oxygen generation. J Am Chem Soc 137:11376–11382
Pang JB, Bachmatiuk A, Yin Y, Trzebicka B, Zhao L, Fu L, Mendes RG, Gemming T, Liu ZF, Rummeli MH (2017) Applications of phosphorene and black phosphorus in energy conversion and storage devices. Adv Energy Mater 1702093:1–43
Sun ZY, Zhang YQ, Yu H, Yan C, Liu YC, Hong S, Tao HC, Robertson AW, Wang Z, Pádua AAH (2018) New solvent-stabilized few-layer black phosphorus for antibacterial applications. Nanoscale 10:12543–12553
Xiang Y, Xia QL, Luo JH, Liu YP, Peng YD, Wang DW, Nie YZ, Guo GH (2018) Observation of ferromagnetism in black phosphorus nanosheets with high magnetization by liquid exfoliation. Solid State Commun 281:1–5
Wu YQ, Lin YM, Bol AA, Jenkins KA, Xia FN, Farmer DB, Zhu Y, Avouris P (2011) High-frequency, scaled graphene transistors on diamond-like carbon. Nature 472:74–78
Feng CP, Bai L, Bao RY, Liu ZY, Yang MB, Chen J, Yang W (2018) Electrically insulating POE/BN elastomeric composites with high through-plane thermal conductivity fabricated by two-roll milling and hot compression. Adv Compos Hybrid Mater 1:160–167
Das S, Chen HY, Penumatcha AV, Appenzeller J (2013) High performance multilayer MoS2 transistors with scandium contacts. Nano Lett 13:100–105
Island JO, Steele GA, van der ZHSJ, Castellanos-Gomez A (2015) Environmental instability of few-layer black phosphorus. 2D Mater 2:011002
Wood JD, Wells SA, Jariwala D, Chen KS, Cho E, Sangwan VK, Liu XL, Lauhon LJ, Marks TJ, Hersam MC (2014) Effective passivation of exfoliated black phosphorus transistors against ambient degradation. Nano Lett 14:6964–6970
Avsar A, Vera-Marun IJ, Tan JY, Watanabe K, Taniguchi T, Castro Neto AH, Özyilmaz B (2015) Air-stable transport in graphene-contacted, fully encapsulated ultrathin black phosphorus-based field-effect transistors. ACS Nano 9:4138–4145
Groenendaal LB, Jonas F, Freitag D, Pielartzik H, Reynolds JR (2000) Poly(3,4-ethylenedioxythiophene) and its derivatives: past, present, and future. Adv Mater 12:481–494
Xu JJ, Peng R, Ran Q, Xian YZ, Tian Y, Jin LT (2010) A highly soluble poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonic acid)/au nanocomposite for horseradish peroxidase immobilization and biosensing. Talanta 82:1511–1515
Lee JY, Lin YJ (2016) Effect of incorporation of black phosphorus into PEDOT:PSS on conductivity and electron-phonon coupling. Synthetic Met 212:180–185
Harano K, Okada S, Furukawa S, Tanaka H, Nakamura E (2014) Formation of a polycrystalline film of donor material on PEDOT:PSS buffer induced by crystal nucleation. J Polym Sci Pol Phys 52:833–841
Niu XL, Weng WJ, Yin CX, Niu YY, Li GJ, Dong RX, Men YL, Sun W (2018) Black phosphorene modified glassy carbon electrode for the sensitive voltammetric detection of rutin. J Electroanal Chem 811:78–83
Li XY, Niu XL, Zhao WS, Chen W, Yin CX, Men YL, Li GJ, Sun W (2018) Black phosphorene and PEDOT:PSS-modified electrode for electrochemistry of hemoglobin. Electrochem Commun 86:68–71
Shi F, Zheng WZ, Wang WC, Hou F, Lei BX, Sun ZF, Sun W (2015) Application of graphene-copper sulfide nanocomposite modified electrode for electrochemistry and electrocatalysis of hemoglobin. Biosens Bioelectron 64:131–137
Qian DP, Li WB, Chen FT, Huang Y, Bao N, Gu HY, Yu CM (2017) Voltammetric sensor for trichloroacetic acid using a glassy carbon electrode modified with au@ag nanorods and hemoglobin. Microchim Acta 184(7):1977–1985
Ranjani B, Kalaiyarasi J, Pavithra L, Devasena T, Pandian K, Gopinath SC (2018) Amperometric determination of nitrite using natural fibers as template for titanium dioxide nanotubes with immobilized hemin as electron transfer mediator. Microchim Acta 185(3):185–194
Wang XF, You Z, Cheng Y, Sha HL, Li GJ, Zhu HH, Sun W (2015) Application of nanosized gold and graphene modified carbon ionic liquid electrode for the sensitive electrochemical determination of folic acid. J Mol Liq 204:112–117
Sun W, Wang XL, Lu YX, Gong SX, Qi XW, Lei BX, Sun ZF, Li GJ (2015) Electrochemical deoxyribonucleic acid biosensor based on electrodeposited graphene and nickel oxide nanoparticle modified electrode for the detection of salmonella enteritidis gene sequence. Mat Sci Eng C-Mater 49:34–39
Zheng W, Chen W, Weng WJ, Liu L, Li GJ, Wang JW, Sun W (2017) Direct electron transfer of horseradish peroxidase at Co3O4-graphene nanocomposite modified electrode and electrocatalysis. J Iran Chem Soc 14:925–932
Guo CX, Hu FP, Li CM, Shen PK (2008) Direct electrochemistry of hemoglobin on carbonized titania nanotubes and its application in a sensitive reagentless hydrogen peroxide biosensor. Biosens Bioelectron 24:819–824
Zhang J, Ding WC, Zhang ZX, Xu JK, Wen YP (2016) Preparation of black phosphorus-PEDOT:PSS hybrid semiconductor composites with good film-forming properties and environmental stability in water containing oxygen. RSC Adv 6:76174–76182
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101:19–28
Bard AJ, Faulkner LR (1980) Electrochemical methods: fundamentals and applications. Wiley, New York
Mimica D, Zagal JH, Bedioui F (2001) Electroreduction of nitrite by hemin, myoglobin and hemoglobin in surfactant films. J Electroanal Chem 497:106–113
Santos WDJR, Lima PR, Tarley CRT, Höehr NF, Kubota LT (2009) Synthesis and application of a peroxidase-like molecularly imprinted polymer based on hemin for selective determination of serotonin in blood serum. Anal Chim Acta 631:170–176
Zhang Y, Xia Z, Liu H, Yang MJ, Lin LL, Li QZ (2013) Hemin-graphene oxide-pristine carbon nanotubes complexes with intrinsic peroxidase-like activity for the detection of H2O2 and simultaneous determination for Trp, AA, DA, and UA. Sens Actuator B 188:496–501
Bhat HK, Kanz MF, Campbell GA, Ansari GAS (1991) Ninety day toxicity study of chloroacetic acids in rats. Fundam Appl Toxicol 17:240–253
Wang WC, Li XQ, Yu XH, Yan LJ, Lei BX, Li P, Chen CX, Sun W (2016) Electrochemistry and electrocatalysis of myoglobin on electrodeposited ZrO2 and graphene modified carbon ionic liquid electrode. J Iran Chem Soc 13:323–330
Li J, Tan SN, Ge HL (1996) Silica sol-gel immobilized amperometric biosensor for hydrogen peroxide. Anal Chim Acta 335:137–145
Zhan TR, Wang XJ, Li XJ, Song Y, Hou WG (2016) Hemoglobin immobilized in exfoliated Co2Al LDH-graphene nanocomposite film: direct electrochemistry and electrocatalysis toward trichloroacetic acid. Sensor Actuat B-Chem 228:101–108
Wang WC, Yan LJ, Shi F, Niu XL, Huang GL, Zheng CJ, Sun W (2016) Application of carbon-microsphere-modified electrodes for electrochemistry of hemoglobin and Electrocatalytic sensing of Trichloroacetic acid. Sensors 16:6
Zhu ZH, Li X, Wang Y, Zeng Y, Sun W, Huang XT (2010) Direct electrochemistry and electrocatalysis of horseradish peroxidase with hyaluronic acid-ionic liquid-cadmium sulfide nanorod composite material. Anal Chim Acta 670:51–56
Sun W, Wang DD, Li GC, Zhai ZQ, Zhao RJ, Jiao K (2008) Direct electron transfer of hemoglobin in a CdS nanorods and Nafion composite film on carbon ionic liquid electrode. Electrochim Acta 53:8217–8221
Ruan CX, Li TT, Niu QJ, Lu M, Lou J, Gao WM, Sun W (2012) Electrochemical myoglobin biosensor based on graphene-ionic liquid-chitosan bionanocomposites: direct electrochemistry and electrocatalysis. Electrochim Acta 64:183–189
Chen W, Weng WJ, Yin CX, Niu XL, Li GJ, Xie H, Liu J, Sun W (2018) Fabrication of an electrochemical biosensor based on Nafion/horseradish peroxidase/Co3O4 NP/CILE and its Electrocatalysis. Int J Electrochem Sci 13:4741–4752
Chen GY, Sun H, Hou SF (2016) Electrochemistry and electrocatalysis of myoglobin immobilized in sulfonated graphene oxide and Nafion films. Anal Biochem 502:43–49
Chen W, Weng WJ, Niu XL, Li XY, Men YL, Sun W, Li GJ, Dong LF (2018) Boron-doped graphene quantum dots modified electrode for electrochemistry and electrocatalysis of hemoglobin. J Electroanal Chem 823:137–145
Niu YY, Xie H, Luo GL, Weng WJ, Ruan CX, Li GJ, Sun W (2019) Electrochemical performance of myoglobin based on TiO2-doped carbon nanofiber decorated electrode and its applications in biosensing. RSC Adv 9:4480–4487
Acknowledgments
This work was financially supported by the National Natural Science Foundation of Hainan Province of China (2017CXTD007), the National Natural Science Foundation of China (21665007), the Program for Innovative Research Team in University (IRT-16R19), Research Fund from Beijing Innovation Center for Future Chips (KYJJ2018006), and the Key Science and Technology Program of Haikou City (2017042).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, X., Luo, G., Xie, H. et al. Voltammetric sensing performances of a carbon ionic liquid electrode modified with black phosphorene and hemin. Microchim Acta 186, 304 (2019). https://doi.org/10.1007/s00604-019-3421-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3421-x